Drug Detail:Cubicin rf (Daptomycin [ dap-to-my-sin ])
Drug Class:
Highlights of Prescribing Information
CUBICIN® RF (daptomycin for injection), for Intravenous Use
Initial U.S. Approval: 2003
Indications and Usage for Cubicin RF
CUBICIN RF is a lipopeptide antibacterial indicated for the treatment of:
- Complicated skin and skin structure infections (cSSSI) in adult and pediatric patients (1 to 17 years of age) (1.1) and,
- Staphylococcus aureus bloodstream infections (bacteremia), in adult patients including those with right-sided infective endocarditis, (1.2)
- Staphylococcus aureus bloodstream infections (bacteremia) in pediatric patients (1 to 17 years of age). (1.3)
Limitations of Use:
- CUBICIN RF is not indicated for the treatment of pneumonia. (1.4)
- CUBICIN RF is not indicated for the treatment of left-sided infective endocarditis due to S. aureus. (1.4)
- CUBICIN RF is not recommended in pediatric patients younger than one year of age due to the risk of potential effects on muscular, neuromuscular, and/or nervous systems (either peripheral and/or central) observed in neonatal dogs. (1.4)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of CUBICIN RF and other antibacterial drugs, CUBICIN RF should be used to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.5)
Cubicin RF Dosage and Administration
Adult Patients
- Administer to adult patients intravenously in 0.9% sodium chloride, either by injection over a 2-minute period or by infusion over a 30-minute period. (2.1, 2.7)
- Recommended dosage regimen for adult patients (2.2, 2.4, 2.6):
| Creatinine Clearance (CLCR) | Dosage Regimen | |
|---|---|---|
| cSSSI
For 7 to 14 days | S. aureus Bacteremia
For 2 to 6 weeks |
|
|
||
| ≥30 mL/min | 4 mg/kg once every 24 hours | 6 mg/kg once every 24 hours |
| <30 mL/min, including hemodialysis and CAPD | 4 mg/kg once every 48 hours* | 6 mg/kg once every 48 hours* |
Pediatric Patients
- Unlike in adults, do NOT administer by injection over a two (2) minute period to pediatric patients. (2.1, 2.7)
- Administer to pediatric patients intravenously in 0.9% sodium chloride, by infusion over a 30- or 60-minute period, based on age. (2.1, 2.7)
- Recommended dosage regimen for pediatric patients (1 to 17 years of age) with cSSSI, based on age (2.3):
| Age group | Dosage* | Duration of therapy |
|---|---|---|
|
||
| 12 to 17 years | 5 mg/kg once every 24 hours infused over 30 minutes | Up to 14 days |
| 7 to 11 years | 7 mg/kg once every 24 hours infused over 30 minutes | |
| 2 to 6 years | 9 mg/kg once every 24 hours infused over 60 minutes | |
| 1 to less than 2 years | 10 mg/kg once every 24 hours infused over 60 minutes | |
- Recommended dosage regimen for pediatric patients (1 to 17 years of age) with S. aureus bacteremia, based on age (2.5):
| Age group | Dosage* | Duration of therapy |
|---|---|---|
|
||
| 12 to 17 years | 7 mg/kg once every 24 hours infused over 30 minutes | Up to 42 days |
| 7 to 11 years | 9 mg/kg once every 24 hours infused over 30 minutes | |
| 1 to 6 years | 12 mg/kg once every 24 hours infused over 60 minutes | |
- There are two formulations of daptomycin that have differences concerning storage and reconstitution. Carefully follow the reconstitution and storage procedures in labeling. (2.7)
- Do not use in conjunction with ReadyMED® elastomeric infusion pumps in adult and pediatric patients. (2.9)
Dosage Forms and Strengths
For Injection: 500 mg lyophilized powder for reconstitution in a single-dose vial (3)
Contraindications
- Known hypersensitivity to daptomycin (4)
Warnings and Precautions
- Anaphylaxis/hypersensitivity reactions (including life-threatening): Discontinue CUBICIN RF and treat signs/symptoms. (5.1)
- Myopathy and rhabdomyolysis: Monitor CPK levels and follow muscle pain or weakness; if elevated CPK or myopathy occurs, consider discontinuation of CUBICIN RF. (5.2)
- Eosinophilic pneumonia: Discontinue CUBICIN RF and consider treatment with systemic steroids. (5.3)
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Discontinue CUBICIN RF and institute appropriate treatment. (5.4)
- Tubulointerstitial Nephritis (TIN): Discontinue CUBICIN RF and institute appropriate treatment. (5.5)
- Peripheral neuropathy: Monitor for neuropathy and consider discontinuation. (5.6)
- Potential nervous system and/or muscular system effects in pediatric patients younger than 12 months: Avoid use of CUBICIN RF in this age group. (5.7)
- Clostridioides difficile–associated diarrhea: Evaluate patients if diarrhea occurs. (5.8)
- Persisting or relapsing S. aureus bacteremia/endocarditis: Perform susceptibility testing and rule out sequestered foci of infection. (5.9)
- Decreased efficacy was observed in adult patients with moderate baseline renal impairment. (5.10)
Adverse Reactions/Side Effects
- Adult cSSSI Patients: The most common adverse reactions that occurred in ≥2% of adult cSSSI patients receiving CUBICIN 4 mg/kg were diarrhea, headache, dizziness, rash, abnormal liver function tests, elevated creatine phosphokinase (CPK), urinary tract infections, hypotension, and dyspnea. (6.1)
- Pediatric cSSSI Patients: The most common adverse reactions that occurred in ≥2% of pediatric patients receiving CUBICIN were diarrhea, vomiting, abdominal pain, pruritus, pyrexia, elevated CPK, and headache. (6.1)
- Adult S. aureus bacteremia/endocarditis Patients: The most common adverse reactions that occurred in ≥5% of S. aureus bacteremia/endocarditis patients receiving CUBICIN 6 mg/kg were sepsis, bacteremia, abdominal pain, chest pain, edema, pharyngolaryngeal pain, pruritus, increased sweating, insomnia, elevated CPK, and hypertension. (6.1)
- Pediatric S. aureus bacteremia Patients: The most common adverse reactions that occurred in ≥5% of pediatric patients receiving CUBICIN were vomiting and elevated CPK. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2022
Related/similar drugs
amoxicillin, doxycycline, ciprofloxacin, cephalexin, metronidazole, azithromycin, clindamycinFull Prescribing Information
1. Indications and Usage for Cubicin RF
1.1 Complicated Skin and Skin Structure Infections (cSSSI)
CUBICIN® RF is indicated for the treatment of adult and pediatric patients (1 to 17 years of age) with complicated skin and skin structure infections (cSSSI) caused by susceptible isolates of the following Gram-positive bacteria: Staphylococcus aureus (including methicillin-resistant isolates), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae subsp. equisimilis, and Enterococcus faecalis (vancomycin-susceptible isolates only).
1.2 Staphylococcus aureus Bloodstream Infections (Bacteremia) in Adult Patients, Including Those with Right-Sided Infective Endocarditis, Caused by Methicillin-Susceptible and Methicillin-Resistant Isolates
CUBICIN RF is indicated for the treatment of adult patients with Staphylococcus aureus bloodstream infections (bacteremia), including adult patients with right-sided infective endocarditis, caused by methicillin-susceptible and methicillin-resistant isolates.
1.3 Staphylococcus aureus Bloodstream Infections (Bacteremia) in Pediatric Patients (1 to 17 Years of Age)
CUBICIN RF is indicated for the treatment of pediatric patients (1 to 17 years of age) with Staphylococcus aureus bloodstream infections (bacteremia).
1.4 Limitations of Use
CUBICIN RF is not indicated for the treatment of pneumonia.
CUBICIN RF is not indicated for the treatment of left-sided infective endocarditis due to S. aureus. The clinical trial of CUBICIN in adult patients with S. aureus bloodstream infections included limited data from patients with left-sided infective endocarditis; outcomes in these patients were poor [see Clinical Studies (14.2)]. CUBICIN has not been studied in patients with prosthetic valve endocarditis.
CUBICIN RF is not recommended in pediatric patients younger than 1 year of age due to the risk of potential effects on muscular, neuromuscular, and/or nervous systems (either peripheral and/or central) observed in neonatal dogs [see Warnings and Precautions (5.7) and Nonclinical Toxicology (13.2)].
1.5 Usage
Appropriate specimens for microbiological examination should be obtained in order to isolate and identify the causative pathogens and to determine their susceptibility to daptomycin.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of CUBICIN RF and other antibacterial drugs, CUBICIN RF should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
When culture and susceptibility information is available, it should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. Empiric therapy may be initiated while awaiting test results.
2. Cubicin RF Dosage and Administration
2.2 Dosage in Adults for cSSSI
Administer CUBICIN RF 4 mg/kg to adult patients intravenously once every 24 hours for 7 to 14 days.
2.3 Dosage in Pediatric Patients (1 to 17 Years of Age) for cSSSI
The recommended dosage regimens based on age for pediatric patients with cSSSI are shown in Table 1. Administer CUBICIN RF intravenously once every 24 hours for up to 14 days.
| Age Range | Dosage Regimen* | Duration of therapy |
|---|---|---|
|
||
| 12 to 17 years | 5 mg/kg once every 24 hours infused over 30 minutes | Up to 14 days |
| 7 to 11 years | 7 mg/kg once every 24 hours infused over 30 minutes | |
| 2 to 6 years | 9 mg/kg once every 24 hours infused over 60 minutes | |
| 1 to less than 2 years | 10 mg/kg once every 24 hours infused over 60 minutes | |
2.4 Dosage in Adult Patients with Staphylococcus aureus Bloodstream Infections (Bacteremia), Including Those with Right-Sided Infective Endocarditis, Caused by Methicillin-Susceptible and Methicillin-Resistant Isolates
Administer CUBICIN RF 6 mg/kg to adult patients intravenously once every 24 hours for 2 to 6 weeks. There are limited safety data for the use of CUBICIN for more than 28 days of therapy. In the Phase 3 trial, there were a total of 14 adult patients who were treated with CUBICIN for more than 28 days.
2.5 Dosage in Pediatric Patients (1 to 17 Years of Age) with Staphylococcus aureus Bloodstream Infections (Bacteremia)
The recommended dosage regimens based on age for pediatric patients with S. aureus bloodstream infections (bacteremia) are shown in Table 2. Administer CUBICIN RF intravenously in 0.9% sodium chloride injection once every 24 hours for up to 42 days.
| Age group | Dosage* | Duration of therapy |
|---|---|---|
|
||
| 12 to 17 years | 7 mg/kg once every 24 hours infused over 30 minutes | Up to 42 days |
| 7 to 11 years | 9 mg/kg once every 24 hours infused over 30 minutes | |
| 1 to 6 years | 12 mg/kg once every 24 hours infused over 60 minutes | |
2.6 Dosage in Patients with Renal Impairment
Adult Patients:
No dosage adjustment is required in adult patients with creatinine clearance (CLCR) greater than or equal to 30 mL/min. The recommended dosage regimen for CUBICIN RF in adult patients with CLCR less than 30 mL/min, including adult patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD), is 4 mg/kg (cSSSI) or 6 mg/kg (S. aureus bloodstream infections) once every 48 hours (Table 3). When possible, CUBICIN RF should be administered following the completion of hemodialysis on hemodialysis days [see Warnings and Precautions (5.2, 5.10), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
| Creatinine Clearance (CLCR) | Dosage Regimen in Adults | |
|---|---|---|
| cSSSI | S. aureus Bloodstream Infections | |
|
||
| Greater than or equal to 30 mL/min | 4 mg/kg once every 24 hours | 6 mg/kg once every 24 hours |
| Less than 30 mL/min, including hemodialysis and CAPD | 4 mg/kg once every 48 hours* | 6 mg/kg once every 48 hours* |
2.7 Preparation and Administration of CUBICIN RF
There are other formulations of daptomycin that have differences concerning reconstitution and storage. Carefully follow the reconstitution and storage procedures described in this labeling.
Administration Instructions
Parenteral drug products should be inspected visually for particulate matter prior to administration.
Slowly remove reconstituted liquid (50 mg daptomycin/mL) from the vial using a beveled sterile needle that is 21 gauge or smaller in diameter. Administer as an intravenous injection or infusion as described below:
Pediatric Patients (1 to 17 Years of Age)
Intravenous Infusion over a period of 30 or 60 minutes
- Unlike in Adults, do NOT administer CUBICIN RF by injection over a two (2) minute period to pediatric patients [see Dosage and Administration (2.1)].
- For Intravenous infusion over a period of 60 minutes in pediatric patients 1 to 6 years of age: The appropriate volume of the reconstituted CUBICIN RF (concentration of 50 mg/mL) should be further diluted, using aseptic technique, into an intravenous infusion bag containing 25 mL of 0.9% sodium chloride injection. The infusion rate should be maintained at 0.42 mL/minute over the 60-minute period.
- For Intravenous infusion over a period of 30 minutes in pediatric patients 7 to 17 years of age: The appropriate volume of the reconstituted CUBICIN RF (concentration of 50 mg/mL) should be further diluted, using aseptic technique, into a 50 mL IV infusion bag containing 0.9% sodium chloride injection. The infusion rate should be maintained at 1.67 mL/minute over the 30-minute period.
No preservative or bacteriostatic agent is present in this product. Aseptic technique must be used in the preparation of final IV solution. Table 4 below provides in-use storage conditions for reconstituted CUBICIN RF in acceptable intravenous diluents in the syringe, vial and intravenous bag (for reconstitution and dilution). Do not exceed the listed shelf-life of reconstituted and diluted solutions of CUBICIN RF. Discard unused portions of CUBICIN RF.
| Container | Diluent | In-Use Shelf-Life | |
|---|---|---|---|
| Room Temperature (20°C–25°C, 68°F–77°F) | Refrigerated (2°C–8°C, 36°F–46°F) |
||
|
|||
| Vial | Sterile Water for Injection | 1 Day | 3 Days |
| Bacteriostatic Water for Injection | 2 Days | 3 Days | |
| Syringe* | Sterile Water for Injection | 1 Day | 3 Days |
| Bacteriostatic Water for Injection | 2 Days | 5 Days | |
| Intravenous Bag | Vial reconstituted with Sterile Water for Injection and immediately diluted with 0.9% sodium chloride injection | 19 Hours | 3 Days |
| Vial reconstituted with Bacteriostatic Water for Injection and immediately diluted with 0.9% sodium chloride injection | 2 Days | 5 Days | |
2.8 Compatible Intravenous Solutions
Reconstituted CUBICIN RF is compatible with Sterile Water for Injection, Bacteriostatic Water for Injection, and 0.9% sodium chloride injection. [See Dosage and Administration (2.7).]
2.9 Incompatibilities
CUBICIN RF is not compatible with dextrose-containing diluents.
CUBICIN RF should not be used in conjunction with ReadyMED® elastomeric infusion pumps. Stability studies of CUBICIN solutions stored in ReadyMED® elastomeric infusion pumps identified an impurity (2-mercaptobenzothiazole) leaching from this pump system into the CUBICIN solution.
Because only limited data are available on the compatibility of CUBICIN RF with other IV substances, additives and other medications should not be added to CUBICIN RF single-dose vials or infusion bags or infused simultaneously with CUBICIN RF through the same IV line. If the same IV line is used for sequential infusion of different drugs, the line should be flushed with a compatible intravenous solution before and after infusion with CUBICIN RF.
3. Dosage Forms and Strengths
For Injection: 500 mg daptomycin as a sterile, pale yellow to light brown lyophilized powder for reconstitution in a single-dose vial.
4. Contraindications
CUBICIN RF is contraindicated in patients with known hypersensitivity to daptomycin [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Anaphylaxis/Hypersensitivity Reactions
Anaphylaxis/hypersensitivity reactions have been reported with the use of antibacterial agents, including CUBICIN, and may be life-threatening. If an allergic reaction to CUBICIN RF occurs, discontinue the drug and institute appropriate therapy [see Adverse Reactions (6.2)].
5.2 Myopathy and Rhabdomyolysis
Myopathy, defined as muscle aching or muscle weakness in conjunction with increases in creatine phosphokinase (CPK) values to greater than 10 times the upper limit of normal (ULN), has been reported with the use of CUBICIN. Rhabdomyolysis, with or without acute renal failure, has been reported [see Adverse Reactions (6.2)].
Patients receiving CUBICIN RF should be monitored for the development of muscle pain or weakness, particularly of the distal extremities. In patients who receive CUBICIN RF, CPK levels should be monitored weekly, and more frequently in patients who received recent prior or concomitant therapy with an HMG-CoA reductase inhibitor or in whom elevations in CPK occur during treatment with CUBICIN RF.
In adult patients with renal impairment, both renal function and CPK should be monitored more frequently than once weekly [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
In Phase 1 studies and Phase 2 clinical trials in adults, CPK elevations appeared to be more frequent when CUBICIN was dosed more than once daily. Therefore, CUBICIN RF should not be dosed more frequently than once a day.
CUBICIN RF should be discontinued in patients with unexplained signs and symptoms of myopathy in conjunction with CPK elevations to levels >1,000 U/L (~5× ULN), and in patients without reported symptoms who have marked elevations in CPK, with levels >2,000 U/L (≥10× ULN).
In addition, consideration should be given to suspending agents associated with rhabdomyolysis, such as HMG-CoA reductase inhibitors, temporarily in patients receiving CUBICIN RF [see Drug Interactions (7.1)].
5.3 Eosinophilic Pneumonia
Eosinophilic pneumonia has been reported in patients receiving CUBICIN [see Adverse Reactions (6.2)]. In reported cases associated with CUBICIN, patients developed fever, dyspnea with hypoxic respiratory insufficiency, and diffuse pulmonary infiltrates or organizing pneumonia. In general, patients developed eosinophilic pneumonia 2 to 4 weeks after starting CUBICIN and improved when CUBICIN was discontinued and steroid therapy was initiated. Recurrence of eosinophilic pneumonia upon re-exposure has been reported. Patients who develop these signs and symptoms while receiving CUBICIN RF should undergo prompt medical evaluation, and CUBICIN RF should be discontinued immediately. Treatment with systemic steroids is recommended.
5.4 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
DRESS has been reported in post-marketing experience with CUBICIN [see Adverse Reactions (6.2)]. Patients who develop skin rash, fever, peripheral eosinophilia, and systemic organ (for example, hepatic, renal, pulmonary) impairment while receiving CUBICIN RF should undergo medical evaluation. If DRESS is suspected, discontinue CUBICIN RF promptly and institute appropriate treatment.
5.5 Tubulointerstitial Nephritis (TIN)
TIN has been reported in post-marketing experience with CUBICIN [see Adverse Reactions (6.2)]. Patients who develop new or worsening renal impairment while receiving CUBICIN RF should undergo medical evaluation. If TIN is suspected, discontinue CUBICIN RF promptly and institute appropriate treatment.
5.6 Peripheral Neuropathy
Cases of peripheral neuropathy have been reported during the CUBICIN postmarketing experience [see Adverse Reactions (6.2)]. Therefore, physicians should be alert to signs and symptoms of peripheral neuropathy in patients receiving CUBICIN RF. Monitor for neuropathy and consider discontinuation.
5.7 Potential Nervous System and/or Muscular System Effects in Pediatric Patients Younger than 12 Months
Avoid use of CUBICIN RF in pediatric patients younger than 12 months due to the risk of potential effects on muscular, neuromuscular, and/or nervous systems (either peripheral and/or central) observed in neonatal dogs with intravenous daptomycin [see Nonclinical Toxicology (13.2)].
5.8 Clostridioides difficile-Associated Diarrhea
Clostridioides difficile–associated diarrhea (CDAD) has been reported with the use of nearly all systemic antibacterial agents, including CUBICIN, and may range in severity from mild diarrhea to fatal colitis [see Adverse Reactions (6.2)]. Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, since these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.9 Persisting or Relapsing S. aureus Bacteremia/Endocarditis
Patients with persisting or relapsing S. aureus bacteremia/endocarditis or poor clinical response should have repeat blood cultures. If a blood culture is positive for S. aureus, minimum inhibitory concentration (MIC) susceptibility testing of the isolate should be performed using a standardized procedure, and diagnostic evaluation of the patient should be performed to rule out sequestered foci of infection. Appropriate surgical intervention (e.g., debridement, removal of prosthetic devices, valve replacement surgery) and/or consideration of a change in antibacterial regimen may be required.
Failure of treatment due to persisting or relapsing S. aureus bacteremia/endocarditis may be due to reduced daptomycin susceptibility (as evidenced by increasing MIC of the S. aureus isolate) [see Clinical Studies (14.2)].
5.10 Decreased Efficacy in Patients with Moderate Baseline Renal Impairment
Limited data are available from the two Phase 3 complicated skin and skin structure infection (cSSSI) trials regarding clinical efficacy of CUBICIN treatment in adult patients with creatinine clearance (CLCR) <50 mL/min; only 31/534 (6%) patients treated with CUBICIN in the intent-to-treat (ITT) population had a baseline CLCR <50 mL/min. Table 5 shows the number of adult patients by renal function and treatment group who were clinical successes in the Phase 3 cSSSI trials.
| CLCR | Success Rate n/N (%) |
|
|---|---|---|
| CUBICIN 4 mg/kg every 24h | Comparator | |
| 50-70 mL/min | 25/38 (66%) | 30/48 (63%) |
| 30-<50 mL/min | 7/15 (47%) | 20/35 (57%) |
In a subgroup analysis of the ITT population in the Phase 3 S. aureus bacteremia/endocarditis trial, clinical success rates, as determined by a treatment-blinded Adjudication Committee [see Clinical Studies (14.2)], in the CUBICIN-treated adult patients were lower in patients with baseline CLCR <50 mL/min (see Table 6). A decrease of the magnitude shown in Table 6 was not observed in comparator-treated patients.
| Baseline CLCR | Success Rate n/N (%) |
|||
|---|---|---|---|---|
| CUBICIN 6 mg/kg every 24h | Comparator | |||
| Bacteremia | Right-Sided Infective Endocarditis | Bacteremia | Right-Sided Infective Endocarditis |
|
| >80 mL/min | 30/50 (60%) | 7/14 (50%) | 19/42 (45%) | 5/11 (46%) |
| 50–80 mL/min | 12/26 (46%) | 1/4 (25%) | 13/31 (42%) | 1/2 (50%) |
| 30–<50 mL/min | 2/14 (14%) | 0/1 (0%) | 7/17 (41%) | 1/1 (100%) |
Consider these data when selecting antibacterial therapy for use in adult patients with baseline moderate to severe renal impairment.
5.11 Increased International Normalized Ratio (INR)/Prolonged Prothrombin Time
Clinically relevant plasma concentrations of daptomycin have been observed to cause a significant concentration-dependent false prolongation of prothrombin time (PT) and elevation of International Normalized Ratio (INR) when certain recombinant thromboplastin reagents are utilized for the assay [see Drug Interactions (7.2)].
6. Adverse Reactions/Side Effects
The following adverse reactions are described, or described in greater detail, in other sections:
- Anaphylaxis/Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Myopathy and Rhabdomyolysis [see Warnings and Precautions (5.2)]
- Eosinophilic Pneumonia [see Warnings and Precautions (5.3)]
- Drug Reaction with Eosinophilia and Systemic Symptoms [see Warnings and Precautions (5.4)]
- Tubulointerstitial Nephritis [see Warnings and Precautions (5.5)]
- Peripheral Neuropathy [see Warnings and Precautions (5.6)]
- Increased International Normalized Ratio (INR)/Prolonged Prothrombin Time [see Warnings and Precautions (5.11) and Drug Interactions (7.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trial Experience in Adult Patients
Clinical trials enrolled 1,864 adult patients treated with CUBICIN and 1,416 treated with comparator.
Complicated Skin and Skin Structure Infection Trials in Adults
In Phase 3 complicated skin and skin structure infection (cSSSI) trials in adult patients, CUBICIN was discontinued in 15/534 (2.8%) patients due to an adverse reaction, while comparator was discontinued in 17/558 (3.0%) patients.
The rates of the most common adverse reactions, organized by body system, observed in adult patients with cSSSI (receiving 4 mg/kg CUBICIN) are displayed in Table 7.
| Adverse Reaction | Adult Patients (%) | |
|---|---|---|
| CUBICIN 4 mg/kg (N=534) | Comparator*
(N=558) |
|
|
||
| Gastrointestinal disorders | ||
| Diarrhea | 5.2 | 4.3 |
| Nervous system disorders | ||
| Headache | 5.4 | 5.4 |
| Dizziness | 2.2 | 2.0 |
| Skin/subcutaneous disorders | ||
| Rash | 4.3 | 3.8 |
| Diagnostic investigations | ||
| Abnormal liver function tests | 3.0 | 1.6 |
| Elevated CPK | 2.8 | 1.8 |
| Infections | ||
| Urinary tract infections | 2.4 | 0.5 |
| Vascular disorders | ||
| Hypotension | 2.4 | 1.4 |
| Respiratory disorders | ||
| Dyspnea | 2.1 | 1.6 |
Drug-related adverse reactions (possibly or probably drug-related) that occurred in <1% of adult patients receiving CUBICIN in the cSSSI trials are as follows:
Body as a Whole: fatigue, weakness, rigors, flushing, hypersensitivity
Blood/Lymphatic System: leukocytosis, thrombocytopenia, thrombocytosis, eosinophilia, increased International Normalized Ratio (INR)
Cardiovascular System: supraventricular arrhythmia
Dermatologic System: eczema
Digestive System: abdominal distension, stomatitis, jaundice, increased serum lactate dehydrogenase
Metabolic/Nutritional System: hypomagnesemia, increased serum bicarbonate, electrolyte disturbance
Musculoskeletal System: myalgia, muscle cramps, muscle weakness, arthralgia
Nervous System: vertigo, mental status change, paresthesia
Special Senses: taste disturbance, eye irritation
S. aureus Bacteremia/Endocarditis Trial in Adults
In the S. aureus bacteremia/endocarditis trial involving adult patients, CUBICIN was discontinued in 20/120 (16.7%) patients due to an adverse reaction, while comparator was discontinued in 21/116 (18.1%) patients.
Serious Gram-negative infections (including bloodstream infections) were reported in 10/120 (8.3%) CUBICIN-treated patients and 0/115 comparator-treated patients. Comparator-treated patients received dual therapy that included initial gentamicin for 4 days. Infections were reported during treatment and during early and late follow-up. Gram-negative infections included cholangitis, alcoholic pancreatitis, sternal osteomyelitis/mediastinitis, bowel infarction, recurrent Crohn's disease, recurrent line sepsis, and recurrent urosepsis caused by a number of different Gram-negative bacteria.
The rates of the most common adverse reactions, organized by System Organ Class (SOC), observed in adult patients with S. aureus bacteremia/endocarditis (receiving 6 mg/kg CUBICIN) are displayed in Table 8.
| Adverse Reaction* | Adult Patients n (%) |
|
|---|---|---|
| CUBICIN 6 mg/kg (N=120) | Comparator†
(N=116) |
|
|
||
| Infections and infestations | ||
| Sepsis NOS | 6 (5%) | 3 (3%) |
| Bacteremia | 6 (5%) | 0 (0%) |
| Gastrointestinal disorders | ||
| Abdominal pain NOS | 7 (6%) | 4 (3%) |
| General disorders and administration site conditions | ||
| Chest pain | 8 (7%) | 7 (6%) |
| Edema NOS | 8 (7%) | 5 (4%) |
| Respiratory, thoracic and mediastinal disorders | ||
| Pharyngolaryngeal pain | 10 (8%) | 2 (2%) |
| Skin and subcutaneous tissue disorders | ||
| Pruritus | 7 (6%) | 6 (5%) |
| Sweating increased | 6 (5%) | 0 (0%) |
| Psychiatric disorders | ||
| Insomnia | 11 (9%) | 8 (7%) |
| Investigations | ||
| Blood creatine phosphokinase increased | 8 (7%) | 1 (1%) |
| Vascular disorders | ||
| Hypertension NOS | 7 (6%) | 3 (3%) |
The following reactions, not included above, were reported as possibly or probably drug-related in the CUBICIN-treated group:
Blood and Lymphatic System Disorders: eosinophilia, lymphadenopathy, thrombocythemia, thrombocytopenia
Cardiac Disorders: atrial fibrillation, atrial flutter, cardiac arrest
Ear and Labyrinth Disorders: tinnitus
Eye Disorders: vision blurred
Gastrointestinal Disorders: dry mouth, epigastric discomfort, gingival pain, hypoesthesia oral
Infections and Infestations: candidal infection NOS, vaginal candidiasis, fungemia, oral candidiasis, urinary tract infection fungal
Investigations: blood phosphorous increased, blood alkaline phosphatase increased, INR increased, liver function test abnormal, alanine aminotransferase increased, aspartate aminotransferase increased, prothrombin time prolonged
Metabolism and Nutrition Disorders: appetite decreased NOS
Musculoskeletal and Connective Tissue Disorders: myalgia
Nervous System Disorders: dyskinesia, paresthesia
Psychiatric Disorders: hallucination NOS
Renal and Urinary Disorders: proteinuria, renal impairment NOS
Skin and Subcutaneous Tissue Disorders: pruritus generalized, rash vesicular
Laboratory Changes in Adults
Complicated Skin and Skin Structure Infection Trials in Adults
In Phase 3 cSSSI trials of adult patients receiving CUBICIN at a dose of 4 mg/kg, elevations in CPK were reported as clinical adverse events in 15/534 (2.8%) CUBICIN-treated patients, compared with 10/558 (1.8%) comparator-treated patients. Of the 534 patients treated with CUBICIN, 1 (0.2%) had symptoms of muscle pain or weakness associated with CPK elevations to greater than 4 times the upper limit of normal (ULN). The symptoms resolved within 3 days and CPK returned to normal within 7 to 10 days after treatment was discontinued [see Warnings and Precautions (5.2)]. Table 9 summarizes the CPK shifts from Baseline through End of Therapy in the cSSSI adult trials.
| Change in CPK | All Adult Patients | Adult Patients with Normal CPK at Baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CUBICIN 4 mg/kg (N=430) | Comparator*
(N=459) | CUBICIN 4 mg/kg (N=374) | Comparator*
(N=392) |
||||||
| % | n | % | n | % | n | % | n | ||
| Note: Elevations in CPK observed in adult patients treated with CUBICIN or comparator were not clinically or statistically significantly different. | |||||||||
|
|||||||||
| No Increase | 90.7 | 390 | 91.1 | 418 | 91.2 | 341 | 91.1 | 357 | |
| Maximum Value | >1× ULN† | 9.3 | 40 | 8.9 | 41 | 8.8 | 33 | 8.9 | 35 |
| >2× ULN | 4.9 | 21 | 4.8 | 22 | 3.7 | 14 | 3.1 | 12 | |
| >4× ULN | 1.4 | 6 | 1.5 | 7 | 1.1 | 4 | 1.0 | 4 | |
| >5× ULN | 1.4 | 6 | 0.4 | 2 | 1.1 | 4 | 0.0 | 0 | |
| >10× ULN | 0.5 | 2 | 0.2 | 1 | 0.2 | 1 | 0.0 | 0 | |
Clinical Trial Experience in Pediatric Patients
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of CUBICIN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: anemia, thrombocytopenia
General and administration site conditions: pyrexia
Immune System Disorders: anaphylaxis; hypersensitivity reactions, including angioedema, pruritus, hives, shortness of breath, difficulty swallowing, truncal erythema, and pulmonary eosinophilia [see Contraindications (4) and Warnings and Precautions (5.1)]
Infections and Infestations: Clostridioides difficile–associated diarrhea [see Warnings and Precautions (5.8)]
Laboratory Investigations: platelet count decreased
Musculoskeletal Disorders: myoglobin increased; rhabdomyolysis (some reports involved patients treated concurrently with CUBICIN and HMG-CoA reductase inhibitors) [see Warnings and Precautions (5.2), Drug Interactions (7.1), and Clinical Pharmacology (12.3)]
Respiratory, Thoracic, and Mediastinal Disorders: cough, eosinophilic pneumonia, organizing pneumonia [see Warnings and Precautions (5.3)]
Nervous System Disorders: peripheral neuropathy [see Warnings and Precautions (5.6)]
Skin and Subcutaneous Tissue Disorders: serious skin reactions, including drug reaction with eosinophilia and systemic symptoms (DRESS), vesiculobullous rash (with or without mucous membrane involvement, including Stevens-Johnson syndrome [SJS] and toxic epidermal necrolysis [TEN]), and acute generalized exanthematous pustulosis [see Warnings and Precautions (5.4)]
Gastrointestinal Disorders: nausea, vomiting
Renal and urinary disorders: acute kidney injury, renal insufficiency, renal failure, and tubulointerstitial nephritis (TIN) [see Warnings and Precautions (5.5)]
Special Senses: visual disturbances
7. Drug Interactions
7.1 HMG-CoA Reductase Inhibitors
In healthy adult subjects, concomitant administration of CUBICIN and simvastatin had no effect on plasma trough concentrations of simvastatin, and there were no reports of skeletal myopathy [see Clinical Pharmacology (12.3)].
However, inhibitors of HMG-CoA reductase may cause myopathy, which is manifested as muscle pain or weakness associated with elevated levels of creatine phosphokinase (CPK). In the adult Phase 3 S. aureus bacteremia/endocarditis trial, some patients who received prior or concomitant treatment with an HMG-CoA reductase inhibitor developed elevated CPK [see Adverse Reactions (6.1)]. Experience with the coadministration of HMG-CoA reductase inhibitors and CUBICIN in patients is limited; therefore, consideration should be given to suspending use of HMG-CoA reductase inhibitors temporarily in patients receiving CUBICIN RF.
7.2 Drug-Laboratory Test Interactions
Clinically relevant plasma concentrations of daptomycin have been observed to cause a significant concentration-dependent false prolongation of prothrombin time (PT) and elevation of International Normalized Ratio (INR) when certain recombinant thromboplastin reagents are utilized for the assay. The possibility of an erroneously elevated PT/INR result due to interaction with a recombinant thromboplastin reagent may be minimized by drawing specimens for PT or INR testing near the time of trough plasma concentrations of daptomycin. However, sufficient daptomycin concentrations may be present at trough to cause interaction.
If confronted with an abnormally high PT/INR result in a patient being treated with CUBICIN RF, it is recommended that clinicians:
- Repeat the assessment of PT/INR, requesting that the specimen be drawn just prior to the next CUBICIN RF dose (i.e., at trough concentration). If the PT/INR value obtained at trough remains substantially elevated above what would otherwise be expected, consider evaluating PT/INR utilizing an alternative method.
- Evaluate for other causes of abnormally elevated PT/INR results.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of CUBICIN RF in the treatment of cSSSI and S. aureus bloodstream infections (bacteremia) have been established in the age groups 1 to 17 years of age. Use of CUBICIN RF in these age groups is supported by evidence from adequate and well-controlled studies in adults, with additional data from pharmacokinetic studies in pediatric patients, and from safety, efficacy and PK studies in pediatric patients with cSSSI and S. aureus bloodstream infections [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1, 14.2)].
Safety and effectiveness in pediatric patients below the age of one year have not been established. Avoid use of CUBICIN RF in pediatric patients younger than one year of age due to the risk of potential effects on muscular, neuromuscular, and/or nervous systems (either peripheral and/or central) observed in neonatal dogs [see Warnings and Precautions (5.7) and Nonclinical Toxicology (13.2)].
CUBICIN RF is not indicated in pediatric patients with renal impairment because dosage has not been established in these patients.
CUBICIN RF has not been studied in pediatric patients with other bacterial infections.
8.5 Geriatric Use
Of the 534 adult patients treated with CUBICIN in Phase 3 controlled clinical trials of complicated skin and skin structure infections (cSSSI), 27% were 65 years of age or older and 12% were 75 years of age or older. Of the 120 adult patients treated with CUBICIN in the Phase 3 controlled clinical trial of S. aureus bacteremia/endocarditis, 25% were 65 years of age or older and 16% were 75 years of age or older. In Phase 3 adult clinical trials of cSSSI and S. aureus bacteremia/endocarditis, clinical success rates were lower in patients ≥65 years of age than in patients <65 years of age. In addition, treatment-emergent adverse events were more common in patients ≥65 years of age than in patients <65 years of age.
The exposure of daptomycin was higher in healthy elderly subjects than in healthy young adult subjects. However, no adjustment of CUBICIN RF dosage is warranted for elderly patients with creatinine clearance (CLCR) ≥30 mL/min [see Dosage and Administration (2.6) and Clinical Pharmacology (12.3)].
8.6 Patients with Renal Impairment
Daptomycin is eliminated primarily by the kidneys; therefore, a modification of CUBICIN RF dosage interval is recommended for adult patients with CLCR <30 mL/min, including patients receiving hemodialysis or continuous ambulatory peritoneal dialysis (CAPD). In adult patients with renal impairment, both renal function and creatine phosphokinase (CPK) should be monitored more frequently than once weekly [see Dosage and Administration (2.6), Warnings and Precautions (5.2, 5.10), and Clinical Pharmacology (12.3)].
The dosage regimen for CUBICIN RF in pediatric patients with renal impairment has not been established.
10. Overdosage
In the event of overdosage, supportive care is advised with maintenance of glomerular filtration. Daptomycin is cleared slowly from the body by hemodialysis (approximately 15% of the administered dose is removed over 4 hours) and by peritoneal dialysis (approximately 11% of the administered dose is removed over 48 hours). The use of high-flux dialysis membranes during 4 hours of hemodialysis may increase the percentage of dose removed compared with that removed by low-flux membranes.
11. Cubicin RF Description
CUBICIN RF (daptomycin for injection) contains daptomycin, a cyclic lipopeptide antibacterial agent derived from the fermentation of Streptomyces roseosporus. The chemical name is N-decanoyl-L-tryptophyl-D-asparaginyl-L-aspartyl-L-threonylglycyl-L-ornithyl-L-aspartyl-D-alanyl-L-aspartylglycyl-D-seryl-threo-3-methyl-L-glutamyl-3-anthraniloyl-L-alanine ε1-lactone. The chemical structure is:
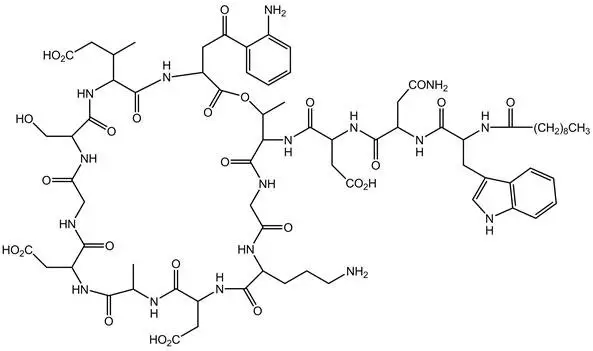
The empirical formula is C72H101N17O26; the molecular weight is 1620.67. CUBICIN RF is supplied in a single-dose vial as a sterile, preservative-free, pale yellow to light brown, lyophilized powder containing 500 mg of daptomycin for intravenous (IV) use following reconstitution [see Dosage and Administration (2.7)]. Each vial also contains 713 mg sucrose and sodium hydroxide is used to adjust the pH. The pH of the solution upon reconstitution is 6.8. Freshly reconstituted solutions of CUBICIN RF range in color from pale yellow to light brown.
12. Cubicin RF - Clinical Pharmacology
12.2 Pharmacodynamics
Based on animal models of infection, the antimicrobial activity of daptomycin appears to correlate with the AUC/MIC (area under the concentration-time curve/minimum inhibitory concentration) ratio for certain pathogens, including S. aureus. The principal pharmacokinetic/pharmacodynamic parameter best associated with clinical and microbiological cure has not been elucidated in clinical trials with CUBICIN RF.
12.3 Pharmacokinetics
CUBICIN Administered over a 30-Minute Period in Adults
The mean and standard deviation (SD) pharmacokinetic parameters of daptomycin at steady-state following intravenous (IV) administration of CUBICIN over a 30-minute period at 4 to 12 mg/kg every 24h to healthy young adults are summarized in Table 12.
| Dose*†
(mg/kg) | Pharmacokinetic Parameters‡ | ||||
|---|---|---|---|---|---|
| AUC0-24
(mcg∙h/mL) | t1/2
(h) | Vss
(L/kg) | CLT
(mL/h/kg) | Cmax
(mcg/mL) |
|
|
|||||
| 4 (N=6) | 494 (75) | 8.1 (1.0) | 0.096 (0.009) | 8.3 (1.3) | 57.8 (3.0) |
| 6 (N=6) | 632 (78) | 7.9 (1.0) | 0.101 (0.007) | 9.1 (1.5) | 93.9 (6.0) |
| 8 (N=6) | 858 (213) | 8.3 (2.2) | 0.101 (0.013) | 9.0 (3.0) | 123.3 (16.0) |
| 10 (N=9) | 1039 (178) | 7.9 (0.6) | 0.098 (0.017) | 8.8 (2.2) | 141.1 (24.0) |
| 12 (N=9) | 1277 (253) | 7.7 (1.1) | 0.097 (0.018) | 9.0 (2.8) | 183.7 (25.0) |
Daptomycin pharmacokinetics were generally linear and time-independent at CUBICIN doses of 4 to 12 mg/kg every 24h administered by IV infusion over a 30-minute period for up to 14 days. Steady-state trough concentrations were achieved by the third daily dose. The mean (SD) steady-state trough concentrations attained following the administration of 4, 6, 8, 10, and 12 mg/kg every 24h were 5.9 (1.6), 6.7 (1.6), 10.3 (5.5), 12.9 (2.9), and 13.7 (5.2) mcg/mL, respectively.
Specific Populations
Patients with Renal Impairment
Population-derived pharmacokinetic parameters were determined for infected adult patients (complicated skin and skin structure infections [cSSSI] and S. aureus bacteremia) and noninfected adult subjects with various degrees of renal function (Table 13). Total plasma clearance (CLT), elimination half-life (t1/2), and volume of distribution at steady-state (Vss) in patients with cSSSI were similar to those in patients with S. aureus bacteremia. Following administration of CUBICIN 4 mg/kg every 24h by IV infusion over a 30-minute period, the mean CLT was 9%, 22%, and 46% lower among subjects and patients with mild (CLCR 50–80 mL/min), moderate (CLCR 30–<50 mL/min), and severe (CLCR <30 mL/min) renal impairment, respectively, than in those with normal renal function (CLCR >80 mL/min). The mean steady-state systemic exposure (AUC), t1/2, and Vss increased with decreasing renal function, although the mean AUC for patients with CLCR 30–80 mL/min was not markedly different from the mean AUC for patients with normal renal function. The mean AUC for patients with CLCR <30 mL/min and for patients on dialysis (CAPD and hemodialysis dosed post-dialysis) was approximately 2 and 3 times higher, respectively, than for patients with normal renal function. The mean Cmax ranged from 60 to 70 mcg/mL in patients with CLCR ≥30 mL/min, while the mean Cmax for patients with CLCR <30 mL/min ranged from 41 to 58 mcg/mL. After administration of CUBICIN 6 mg/kg every 24h by IV infusion over a 30-minute period, the mean Cmax ranged from 80 to 114 mcg/mL in patients with mild to moderate renal impairment and was similar to that of patients with normal renal function.
| Renal Function | Pharmacokinetic Parameters* | |||||
|---|---|---|---|---|---|---|
| t1/2†
(h) 4 mg/kg | Vss†
(L/kg) 4 mg/kg | CLT†
(mL/h/kg) 4 mg/kg | AUC0-∞†
(mcg∙h/mL) 4 mg/kg | AUCss‡
(mcg∙h/mL) 6 mg/kg | Cmin,ss‡
(mcg/mL) 6 mg/kg |
|
| Note: CUBICIN was administered over a 30-minute period. | ||||||
|
||||||
| Normal (CLCR >80 mL/min) | 9.39 (4.74) N=165 | 0.13 (0.05) N=165 | 10.9 (4.0) N=165 | 417 (155) N=165 | 545 (296) N=62 | 6.9 (3.5) N=61 |
| Mild Renal Impairment (CLCR 50–80 mL/min) | 10.75 (8.36) N=64 | 0.12 (0.05) N=64 | 9.9 (4.0) N=64 | 466 (177) N=64 | 637 (215) N=29 | 12.4 (5.6) N=29 |
| Moderate Renal Impairment (CLCR 30–<50 mL/min) | 14.70 (10.50) N=24 | 0.15 (0.06) N=24 | 8.5 (3.4) N=24 | 560 (258) N=24 | 868 (349) N=15 | 19.0 (9.0) N=14 |
| Severe Renal Impairment (CLCR <30 mL/min) | 27.83 (14.85) N=8 | 0.20 (0.15) N=8 | 5.9 (3.9) N=8 | 925 (467) N=8 | 1050 (892) N=2 | 24.4 (21.4) N=2 |
| Hemodialysis | 30.51 (6.51) N=16 | 0.16 (0.04) N=16 | 3.9 (2.1) N=16 | 1193 (399) N=16 | NA | NA |
| CAPD | 27.56 (4.53) N=5 | 0.11 (0.02) N=5 | 2.9 (0.4) N=5 | 1409 (238) N=5 | NA | NA |
Because renal excretion is the primary route of elimination, adjustment of CUBICIN RF dosage interval is necessary in adult patients with severe renal impairment (CLCR <30 mL/min) [see Dosage and Administration (2.6)].
Pediatric Patients
The pharmacokinetics of daptomycin in pediatric subjects was evaluated in 3 single-dose pharmacokinetic studies. In general, body weight-normalized total body clearance in pediatric patients was higher than in adults and increased with a decrease of age, whereas elimination half-life tends to decrease with a decrease of age. Body weight-normalized total body clearance and elimination half-life of daptomycin in children 2 to 6 years of age were similar at different doses.
A study was conducted to assess safety, efficacy, and pharmacokinetics of daptomycin in pediatric patients (1 to 17 years old, inclusive) with cSSSI caused by Gram-positive pathogens. Patients were enrolled into 4 age groups [see Clinical Studies (14.1)], and intravenous CUBICIN doses of 5 to 10 mg/kg once daily were administered. Following administration of multiple doses, daptomycin exposure (AUCss and Cmax,ss) was similar across different age groups after dose adjustment based on body weight and age (Table 14).
| Age | Pharmacokinetic Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | Infusion Duration (min) | AUCss
(mcg∙h/mL) | t1/2
(h) | Vss
(mL) | CLT
(mL/h/kg) | Cmax,ss
(mcg/mL) |
|
| AUCss, area under the concentration-time curve at steady state; CLT, clearance normalized to body weight; Vss, volume of distribution at steady state; t½, terminal half-life | |||||||
|
|||||||
| 12 to 17 years (N=6) | 5 | 30 | 434 (67.9) | 7.1 (0.9) | 8200 (3250) | 11.8 (2.15) | 76.4 (6.75) |
| 7 to 11 years (N=2) | 7 | 30 | 543* | 6.8* | 4470* | 13.2* | 92.4* |
| 2 to 6 years (N=7) | 9 | 60 | 452 (93.1) | 4.6 (0.8) | 2750 (832) | 20.8 (4.29) | 90.3 (14.0) |
| 1 to less than 2 years (N=27) | 10 | 60 | 462 (138) | 4.8 (0.6) | 1670 (446) | 23.1 (5.43) | 81.6 (20.7) |
A study was conducted to assess safety, efficacy, and pharmacokinetics of daptomycin in pediatric patients with S. aureus bacteremia. Patients were enrolled into 3 age groups [see Clinical Studies (14.2)], and intravenous doses of 7 to 12 mg/kg once daily were administered. Following administration of multiple doses, daptomycin exposure (AUCss and Cmax,ss) was similar across different age groups after dose adjustment based on body weight and age (Table 15).
| Age | Pharmacokinetic Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | Infusion Duration (min) | AUCss
(mcg∙h/mL) | t1/2
(h) | Vss
(mL) | CLT
(mL/h/kg) | Cmax,ss
(mcg/mL) |
|
| AUCss, area under the concentration-time curve at steady state; CLT, clearance normalized to body weight; Vss, volume of distribution at steady state; t½, terminal half-life | |||||||
| No patients 1 to <2 years of age were enrolled in the study. Simulation using a population pharmacokinetic model demonstrated that the AUCss of daptomycin in pediatric patients 1 to <2 years of age receiving 12 mg/kg once daily would be comparable to that in adult patients receiving 6 mg/kg once daily. | |||||||
| 12 to 17 years (N=13) | 7 | 30 | 656 (334) | 7.5 (2.3) | 6420 (1980) | 12.4 (3.9) | 104 (35.5) |
| 7 to 11 years (N=19) | 9 | 30 | 579 (116) | 6.0 (0.8) | 4510 (1470) | 15.9 (2.8) | 104 (14.5) |
| 2 to 6 years (N=19) | 12 | 60 | 620 (109) | 5.1 (0.6) | 2200 (570) | 19.9 (3.4) | 106 (12.8) |
12.4 Microbiology
Daptomycin belongs to the cyclic lipopeptide class of antibacterials. Daptomycin has clinical utility in the treatment of infections caused by aerobic, Gram-positive bacteria. The in vitro spectrum of activity of daptomycin encompasses most clinically relevant Gram-positive pathogenic bacteria.
Daptomycin exhibits rapid, concentration-dependent bactericidal activity against Gram-positive bacteria in vitro. This has been demonstrated both by time-kill curves and by MBC/MIC (minimum bactericidal concentration/minimum inhibitory concentration) ratios using broth dilution methodology. Daptomycin maintained bactericidal activity in vitro against stationary phase S. aureus in simulated endocardial vegetations. The clinical significance of this is not known.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in animals have not been conducted to evaluate the carcinogenic potential of CUBICIN. However, neither mutagenic nor clastogenic potential was found in a battery of genotoxicity tests, including the Ames assay, a mammalian cell gene mutation assay, a test for chromosomal aberrations in Chinese hamster ovary cells, an in vivo micronucleus assay, an in vitro DNA repair assay, and an in vivo sister chromatid exchange assay in Chinese hamsters.
Daptomycin did not affect the fertility or reproductive performance of male and female rats when administered intravenously at doses of 25, 75, or 150 mg/kg/day, which is approximately up to 9 times the estimated human exposure level based upon AUCs (or approximately up to 4 times the recommended human dose of 6 mg/kg based on body surface area comparison).
14. Clinical Studies
14.1 Complicated Skin and Skin Structure Infections
Adults with cSSSI
Adult patients with clinically documented complicated skin and skin structure infections (cSSSI) (Table 16) were enrolled in two randomized, multinational, multicenter, investigator-blinded trials comparing CUBICIN (4 mg/kg IV every 24h) with either vancomycin (1 g IV q12h) or an anti-staphylococcal semi-synthetic penicillin (i.e., nafcillin, oxacillin, cloxacillin, or flucloxacillin; 4 to 12 g IV per day). Patients could switch to oral therapy after a minimum of 4 days of IV treatment if clinical improvement was demonstrated. Patients known to have bacteremia at baseline were excluded. Patients with creatinine clearance (CLCR) between 30 and 70 mL/min were to receive a lower dose of CUBICIN as specified in the protocol; however, the majority of patients in this subpopulation did not have the dose of CUBICIN adjusted.
| Primary Diagnosis | Adult Patients (CUBICIN / Comparator*) |
||
|---|---|---|---|
| Study 9801 N=264 / N=266 | Study 9901 N=270 / N=292 | Pooled N=534 / N=558 |
|
|
|||
| Wound Infection | 99 (38%) / 116 (44%) | 102 (38%) / 108 (37%) | 201 (38%) / 224 (40%) |
| Major Abscess | 55 (21%) / 43 (16%) | 59 (22%) / 65 (22%) | 114 (21%) / 108 (19%) |
| Ulcer Infection | 71 (27%) / 75 (28%) | 53 (20%) / 68 (23%) | 124 (23%) / 143 (26%) |
| Other Infection† | 39 (15%) / 32 (12%) | 56 (21%) / 51 (18%) | 95 (18%) / 83 (15%) |
One trial was conducted primarily in the United States and South Africa (study 9801), and the second was conducted at non-US sites only (study 9901). The two trials were similar in design but differed in patient characteristics, including history of diabetes and peripheral vascular disease. There were a total of 534 adult patients treated with CUBICIN and 558 treated with comparator in the two trials. The majority (89.7%) of patients received IV medication exclusively.
The efficacy endpoints in both trials were the clinical success rates in the intent-to-treat (ITT) population and in the clinically evaluable (CE) population. In study 9801, clinical success rates in the ITT population were 62.5% (165/264) in patients treated with CUBICIN and 60.9% (162/266) in patients treated with comparator drugs. Clinical success rates in the CE population were 76.0% (158/208) in patients treated with CUBICIN and 76.7% (158/206) in patients treated with comparator drugs. In study 9901, clinical success rates in the ITT population were 80.4% (217/270) in patients treated with CUBICIN and 80.5% (235/292) in patients treated with comparator drugs. Clinical success rates in the CE population were 89.9% (214/238) in patients treated with CUBICIN and 90.4% (226/250) in patients treated with comparator drugs.
The success rates by pathogen for microbiologically evaluable patients are presented in Table 17.
| Pathogen | Success Rate n/N (%) |
|
|---|---|---|
| CUBICIN | Comparator* | |
|
||
| Methicillin-susceptible Staphylococcus aureus (MSSA)† | 170/198 (86%) | 180/207 (87%) |
| Methicillin-resistant Staphylococcus aureus (MRSA)† | 21/28 (75%) | 25/36 (69%) |
| Streptococcus pyogenes | 79/84 (94%) | 80/88 (91%) |
| Streptococcus agalactiae | 23/27 (85%) | 22/29 (76%) |
| Streptococcus dysgalactiae subsp. equisimilis | 8/8 (100%) | 9/11 (82%) |
| Enterococcus faecalis (vancomycin-susceptible only) | 27/37 (73%) | 40/53 (76%) |
14.2 S. aureus Bacteremia/Endocarditis
Adults with S. aureus Bacteremia/Endocarditis
The efficacy of CUBICIN in the treatment of adult patients with S. aureus bacteremia was demonstrated in a randomized, controlled, multinational, multicenter, open-label trial. In this trial, adult patients with at least one positive blood culture for S. aureus obtained within 2 calendar days prior to the first dose of study drug and irrespective of source were enrolled and randomized to either CUBICIN (6 mg/kg IV every 24h) or standard of care [an anti-staphylococcal semi-synthetic penicillin 2 g IV q4h (nafcillin, oxacillin, cloxacillin, or flucloxacillin) or vancomycin 1 g IV q12h, each with initial gentamicin 1 mg/kg IV every 8 hours for first 4 days]. Of the patients in the comparator group, 93% received initial gentamicin for a median of 4 days, compared with 1 patient (<1%) in the CUBICIN group. Patients with prosthetic heart valves, intravascular foreign material that was not planned for removal within 4 days after the first dose of study medication, severe neutropenia, known osteomyelitis, polymicrobial bloodstream infections, creatinine clearance <30 mL/min, and pneumonia were excluded.
Upon entry, patients were classified for likelihood of endocarditis using the modified Duke criteria (Possible, Definite, or Not Endocarditis). Echocardiography, including a transesophageal echocardiogram (TEE), was performed within 5 days following study enrollment. The choice of comparator agent was based on the oxacillin susceptibility of the S. aureus isolate. The duration of study treatment was based on the investigator's clinical diagnosis. Final diagnoses and outcome assessments at Test of Cure (6 weeks after the last treatment dose) were made by a treatment-blinded Adjudication Committee, using protocol-specified clinical definitions and a composite primary efficacy endpoint (clinical and microbiological success) at the Test of Cure visit.
A total of 246 patients ≥18 years of age (124 CUBICIN, 122 comparator) with S. aureus bacteremia were randomized from 48 centers in the US and Europe. In the ITT population, 120 patients received CUBICIN and 115 received comparator (62 received an anti-staphylococcal semi-synthetic penicillin and 53 received vancomycin). Thirty-five patients treated with an anti-staphylococcal semi-synthetic penicillin received vancomycin initially for 1 to 3 days, pending final susceptibility results for the S. aureus isolates. The median age among the 235 patients in the ITT population was 53 years (range: 21 to 91 years); 30/120 (25%) in the CUBICIN group and 37/115 (32%) in the comparator group were ≥65 years of age. Of the 235 ITT patients, there were 141 (60%) males and 156 (66%) Caucasians across the two treatment groups. In addition, 176 (75%) of the ITT population had systemic inflammatory response syndrome (SIRS) at baseline and 85 (36%) had surgical procedures within 30 days prior to onset of the S. aureus bacteremia. Eighty-nine patients (38%) had bacteremia caused by methicillin-resistant S. aureus (MRSA). Entry diagnosis was based on the modified Duke criteria and comprised 37 (16%) Definite, 144 (61%) Possible, and 54 (23%) Not Endocarditis. Of the 37 patients with an entry diagnosis of Definite Endocarditis, all (100%) had a final diagnosis of infective endocarditis, and of the 144 patients with an entry diagnosis of Possible Endocarditis, 15 (10%) had a final diagnosis of infective endocarditis as assessed by the Adjudication Committee. Of the 54 patients with an entry diagnosis of Not Endocarditis, 1 (2%) had a final diagnosis of infective endocarditis as assessed by the Adjudication Committee.
In the ITT population, there were 182 patients with bacteremia and 53 patients with infective endocarditis as assessed by the Adjudication Committee, including 35 with right-sided endocarditis and 18 with left-sided endocarditis. The 182 patients with bacteremia comprised 121 with complicated S. aureus bacteremia and 61 with uncomplicated S. aureus bacteremia.
Complicated bacteremia was defined as S. aureus isolated from blood cultures obtained on at least 2 different calendar days, and/or metastatic foci of infection (deep tissue involvement), and classification of the patient as not having endocarditis according to the modified Duke criteria. Uncomplicated bacteremia was defined as S. aureus isolated from blood culture(s) obtained on a single calendar day, no metastatic foci of infection, no infection of prosthetic material, and classification of the patient as not having endocarditis according to the modified Duke criteria. The definition of right-sided infective endocarditis (RIE) used in the clinical trial was Definite or Possible Endocarditis according to the modified Duke criteria and no echocardiographic evidence of predisposing pathology or active involvement of either the mitral or aortic valve. Complicated RIE comprised patients who were not intravenous drug users, had a positive blood culture for MRSA, serum creatinine ≥2.5 mg/dL, or evidence of extrapulmonary sites of infection. Patients who were intravenous drug users, had a positive blood culture for methicillin-susceptible S. aureus (MSSA), had serum creatinine <2.5 mg/dL, and were without evidence of extrapulmonary sites of infection were considered to have uncomplicated RIE.
The coprimary efficacy endpoints in the trial were the Adjudication Committee success rates at the Test of Cure visit (6 weeks after the last treatment dose) in the ITT and Per Protocol (PP) populations. The overall Adjudication Committee success rates in the ITT population were 44.2% (53/120) in patients treated with CUBICIN and 41.7% (48/115) in patients treated with comparator (difference = 2.4% [95% CI −10.2, 15.1]). The success rates in the PP population were 54.4% (43/79) in patients treated with CUBICIN and 53.3% (32/60) in patients treated with comparator (difference = 1.1% [95% CI −15.6, 17.8]).
Adjudication Committee success rates are shown in Table 18.
| Population | Success Rate n/N (%) | Difference: CUBICIN−Comparator (Confidence Interval) |
|
|---|---|---|---|
| CUBICIN 6 mg/kg | Comparator* | ||
|
|||
| Overall | 53/120 (44%) | 48/115 (42%) | 2.4% (−10.2, 15.1)† |
| Baseline Pathogen | |||
| Methicillin-susceptible S. aureus | 33/74 (45%) | 34/70 (49%) | −4.0% (−22.6, 14.6)‡ |
| Methicillin-resistant S. aureus | 20/45 (44%) | 14/44 (32%) | 12.6% (−10.2, 35.5)‡ |
| Entry Diagnosis§ | |||
| Definite or Possible Infective Endocarditis | 41/90 (46%) | 37/91 (41%) | 4.9% (−11.6, 21.4)‡ |
| Not Infective Endocarditis | 12/30 (40%) | 11/24 (46%) | −5.8% (−36.2, 24.5)‡ |
| Final Diagnosis | |||
| Uncomplicated Bacteremia | 18/32 (56%) | 16/29 (55%) | 1.1% (−31.7, 33.9)¶ |
| Complicated Bacteremia | 26/60 (43%) | 23/61 (38%) | 5.6% (−17.3, 28.6)¶ |
| Right-Sided Infective Endocarditis | 8/19 (42%) | 7/16 (44%) | −1.6% (−44.9, 41.6)¶ |
| Uncomplicated Right-Sided Infective Endocarditis | 3/6 (50%) | 1/4 (25%) | 25.0% (−51.6, 100.0)¶ |
| Complicated Right-Sided Infective Endocarditis | 5/13 (39%) | 6/12 (50%) | −11.5% (−62.4, 39.4)¶ |
| Left-Sided Infective Endocarditis | 1/9 (11%) | 2/9 (22%) | −11.1% (−55.9, 33.6)¶ |
Eighteen (18/120) patients in the CUBICIN arm and 19/116 patients in the comparator arm died during the trial. These comprise 3/28 CUBICIN-treated patients and 8/26 comparator-treated patients with endocarditis, as well as 15/92 CUBICIN-treated patients and 11/90 comparator-treated patients with bacteremia. Among patients with persisting or relapsing S. aureus infections, 8/19 CUBICIN-treated patients and 7/11 comparator-treated patients died.
Overall, there was no difference in time to clearance of S. aureus bacteremia between CUBICIN and comparator. The median time to clearance in patients with MSSA was 4 days and in patients with MRSA was 8 days.
Failure of treatment due to persisting or relapsing S. aureus infections was assessed by the Adjudication Committee in 19/120 (16%) CUBICIN-treated patients (12 with MRSA and 7 with MSSA) and 11/115 (10%) comparator-treated patients (9 with MRSA treated with vancomycin and 2 with MSSA treated with an anti-staphylococcal semi-synthetic penicillin). Among all failures, isolates from 6 CUBICIN-treated patients and 1 vancomycin-treated patient developed increasing MICs (reduced susceptibility) by central laboratory testing during or following therapy. Most patients who failed due to persisting or relapsing S. aureus infection had deep-seated infection and did not receive necessary surgical intervention [see Warnings and Precautions (5.9)].
15. References
- Liu SL, Howard LC, Van Lier RBL, Markham JK: Teratology studies with daptomycin administered intravenously (iv) to rats and rabbits. Teratology 37(5):475, 1988.
- Stroup JS, Wagner J, Badzinski T: Use of daptomycin in a pregnant patient with Staphylococcus aureus endocarditis. Ann Pharmacother 44(4):746-749, 2010.
- Buitrago MI, Crompton JA, Bertolami S, North DS, Nathan RA. Extremely low excretion of daptomycin into breast milk of a nursing mother with methicillin-resistant Staphylococcus aureus pelvic inflammatory disease. Pharmacotherapy 2009;29(3):347–351.
- Klibanov OM, Vickery S, Nortey C: Successful treatment of infective panniculitis with daptomycin in a pregnant, morbidly obese patient. Ann Pharmacother 48(5):652-655, 2014.
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–638.
16. How is Cubicin RF supplied
CUBICIN RF (daptomycin for injection) is supplied as a sterile pale yellow to light brown lyophilized powder in a single-dose vial containing 500 mg of daptomycin: Package of 1 (NDC 67919-012-01).
17. Patient Counseling Information
Allergic Reactions
Advise patients that allergic reactions, including serious skin, kidney, lung, or other organ reactions, could occur and that these serious reactions require immediate treatment. Patients should report any previous allergic reactions to daptomycin [see Warnings and Precautions (5.1, 5.4, 5.5)].
Muscle Pain or Weakness (Myopathy and Rhabdomyolysis, Peripheral Neuropathy)
Advise patients to report muscle pain or weakness, especially in the forearms and lower legs, as well as tingling or numbness [see Warnings and Precautions (5.2, 5.6)].
Cough, Breathlessness, or Fever (Eosinophilic Pneumonia)
Advise patients to report any symptoms of cough, breathlessness, or fever [see Warnings and Precautions (5.3)].
C. difficile-Associated Diarrhea (CDAD)
Advise patients that diarrhea is a common problem caused by antibacterials, including CUBICIN RF, that usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials, including CUBICIN RF, patients can develop watery and bloody stools (with or without stomach cramps and fever), even as late as 2 or more months after having received the last dose of the antibacterial. If this occurs, patients should contact their physician as soon as possible [see Warnings and Precautions (5.8)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs, including CUBICIN RF, should be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When CUBICIN RF is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be administered exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by CUBICIN RF or other antibacterial drugs in the future.
| CUBICIN RF
daptomycin injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |





