Drug Detail:Cycloset (Bromocriptine [ broe-moe-krip-teen ])
Drug Class: Dopaminergic antiparkinsonism agents Prolactin inhibitors
Highlights of Prescribing Information
CYCLOSET® (bromocriptine mesylate tablets), for oral use
Initial U.S. Approval: 1978
Indications and Usage for Cycloset
CYCLOSET is a dopamine receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1.1, 11)
Important Limitations of Use:
- Should not be used to treat type 1 diabetes or diabetic ketoacidosis. (1.2)
- Limited efficacy data in combination with thiazolidinediones. (1.2)
- Efficacy has not been confirmed in combination with insulin. (1.2)
Cycloset Dosage and Administration
- Taken within two hours after waking in the morning with food (2.1)
- Initial dose is one tablet (0.8 mg) daily increased weekly by one tablet until maximal tolerated daily dose of 1.6 to 4.8 mg is achieved. (2.2)
- Limit dose to 1.6 mg daily during concomitant use of a moderate CYP3A4 inhibitor. Avoid concomitant use with strong CYP3A4 inhibitors. (2.3)
Dosage Forms and Strengths
Tablets: 0.8 mg (3)
Contraindications
- Do not use in patients with hypersensitivity to ergot-related drugs, bromocriptine or to any of the excipients in CYCLOSET. (4)
- Do not use in patients with syncopal migraines. May precipitate hypotension. (4)
- Do not use in nursing women. May inhibit lactation. Postmarketing reports of stroke in this patient population. (4, 6.2, 8.3)
Warnings and Precautions
- Hypotension: Can cause orthostatic hypotension and syncope, particularly upon initiation or dose escalation. Use caution in patients taking antihypertensive medications. Assess orthostatic vital signs prior to initiation of CYCLOSET and periodically thereafter. Advise patients during early treatment to avoid situations that could lead to injury if syncope was to occur. (5.1, 6.1)
- Psychosis: May exacerbate psychotic disorders or reduce the effectiveness of drugs that treat psychosis. Use in patients with severe psychotic disorders is not recommended. (5.2)
- Somnolence: May cause somnolence. Advise patients not to operate heavy machinery if symptoms of somnolence occur. (5.3)
- Interaction with dopamine antagonists: Concomitant use with dopamine antagonists such as neuroleptic agents may diminish the effectiveness of both drugs. Concomitant use is not recommended. (5.4, 7)
- Other dopamine receptor agonists: Effectiveness and safety are unknown in patients already taking dopamine receptor agonists for other indications. Concomitant use is not recommended. (5.5)
- Macrovascular outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with CYCLOSET or any other antidiabetic drug. CYCLOSET does not increase the risk of macrovascular events. (5.6, 6.1)
Adverse Reactions/Side Effects
In controlled clinical trials, adverse reactions reported in ≥5% of patients treated with CYCLOSET and reported more commonly than in patients treated with placebo, included nausea, fatigue, dizziness, vomiting, and headache. (6.1)
Postmarketing reports with higher doses of bromocriptine used for other indications include psychotic disorders, hallucinations, and fibrotic complications. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact VeroScience, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- May increase the unbound fraction of highly protein-bound therapies, altering their effectiveness and safety profiles. (7)
- May increase ergot-related side effects or reduce ergot effectiveness for migraines if co-administered within 6 hours of ergot-related drugs. (7)
- Extensively metabolized by CYP3A4. Limit CYCLOSET dose to 1.6 mg/day during concomitant use of moderate CYP3A4 inhibitors. Avoid concomitant use of CYCLOSET with strong CYP3A4 inhibitors. (2.3, 7)
Use In Specific Populations
Pediatrics: Safety and effectiveness have not been established. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2020
Full Prescribing Information
1. Indications and Usage for Cycloset
2. Cycloset Dosage and Administration
2.1 Recommended Dosing
The recommended dose of CYCLOSET is 1.6 mg to 4.8 mg administered once daily within two hours after waking in the morning. CYCLOSET should be taken with food to potentially reduce gastrointestinal side effects such as nausea.
2.2 Titration
CYCLOSET should be initiated at one tablet (0.8 mg) and increased by one tablet per week until a maximum daily dose of 6 tablets (4.8 mg) or until the maximal tolerated number of tablets between 2 and 6 per day is reached.
2.3 Use with Concomitant Therapy
CYCLOSET dose should not exceed 1.6 mg once daily during concomitant use of a moderate CYP3A4 inhibitor (e.g., erythromycin). Avoid concomitant use of CYCLOSET and strong CYP3A4 inhibitors (e.g., azole antimycotics, HIV protease inhibitors) and ensure adequate washout of the strong CYP3A4 inhibitor drug before initiating CYCLOSET treatment [see Drug Interactions (7), Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
0.8 mg tablets are white and round, imprinted with "C" on one side and "9" on the other.
4. Contraindications
CYCLOSET is contraindicated in:
- Patients with known hypersensitivity to bromocriptine, ergot-related drugs, or any of the excipients in CYCLOSET.
- Patients with syncopal migraine. Bromocriptine increases the likelihood of a hypotensive episode among patients with syncopal migraine. Loss of consciousness during a migraine may reflect dopamine receptor hypersensitivity. CYCLOSET is a dopamine receptor agonist and may, therefore, potentiate the risk for syncope in these patients.
- Women who are nursing their children. CYCLOSET may inhibit lactation. There are postmarketing reports of stroke in this patient population although causality has not been proven [see Use in Specific Populations (8.3)].
5. Warnings and Precautions
5.1 Hypotension
Hypotension, including orthostatic hypotension, can occur, particularly upon initiation of CYCLOSET therapy and with dose escalation. In a 52-week, randomized clinical trial of 3070 patients, hypotension was reported in 2.2% of patients randomized to CYCLOSET compared to 0.8% of patients randomized to placebo. Among CYCLOSET-treated patients reporting symptomatic hypotension, 98% were on at least one blood pressure medication compared to 73% on such medication in the total study population. In this trial, six CYCLOSET-treated patients (0.3%) reported an adverse event of orthostatic hypotension compared to 2 (0.2%) placebo-treated patients. All six patients were taking antihypertensive medications. Hypotension can result in syncope. In this trial, syncope due to any cause was reported in 1.6% of CYCLOSET-treated patients and 0.7% of placebo-treated patients [see Adverse Reactions (6.1)]. As a precaution, assessment of orthostatic vital signs is recommended prior to initiation of CYCLOSET and periodically thereafter. During early treatment with CYCLOSET, patients should be advised to make slow postural changes and to avoid situations that could lead to serious injury if syncope was to occur. Use caution in patients taking antihypertensive medications.
5.2 Psychotic Disorders
In patients with severe psychotic disorders, treatment with a dopamine receptor agonist such as CYCLOSET may exacerbate the disorder or may diminish the effectiveness of drugs used to treat the disorder. Therefore, the use of CYCLOSET in patients with severe psychotic disorders in not recommended.
5.3 Somnolence
CYCLOSET may cause somnolence. In a 52-week, randomized clinical trial, 4.3% of CYCLOSET-treated patients and 1.3% of placebo-treated patients reported somnolence as an adverse event. None of these events were reported as serious, and the majority of patients reported resolution of somnolence over time. Patients should be made aware of this potential side effect, particularly when initiating therapy with CYCLOSET. Patients experiencing somnolence should refrain from driving or operating heavy machinery.
5.4 Interaction with Dopamine Receptor Antagonists
Dopamine receptor antagonists, including neuroleptic agents that have dopamine D2 receptor antagonist properties (e.g., clozapine, olanzapine, ziprasidone), may reduce the effectiveness of CYCLOSET, and CYCLOSET may reduce the effectiveness of these agents. CYCLOSET has not been studied in patients taking neuroleptic drugs. The concomitant use of CYCLOSET and dopamine receptor antagonists, including neuroleptic drugs, is not recommended.
5.5 Other Dopamine Receptor Agonists
Other dopamine receptor agonists are indicated for the treatment of Parkinson's disease, hyperprolactinemia, restless leg syndrome, acromegaly, and other disorders. The effectiveness and safety of CYCLOSET in patients who are already taking one of these other dopamine receptor agonists is unknown. Concomitant use is not recommended.
5.6. Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with CYCLOSET or any other antidiabetic drug. In a 52-week, randomized clinical trial, CYCLOSET use was not associated with an increased risk for adverse cardiovascular events [see Adverse Reactions (6.1)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice.
In the pooled CYCLOSET Phase 3 clinical trials (CYCLOSET N = 2,298; placebo N = 1,266), adverse events leading to discontinuation occurred in 539 (24%) CYCLOSET-treated patients and 118 (9%) placebo-treated patients. This between-group difference was driven mostly by gastrointestinal adverse events, particularly nausea.
The CYCLOSET safety trial was a 52-week, placebo-controlled study that included patients treated only with diet therapy or with other antidiabetic medications. A total of 3,070 patients were randomized to CYCLOSET (titrated to 1.6 to 4.8 mg daily, as tolerated) or placebo. The study population had a mean baseline age of 60 years (range 27-80) and 33% were 65 years of age or older. Approximately 43% of the patients were female, 68% were Caucasian, 17% were Black, 13% were Hispanic, and 1% were Asian. The mean baseline body mass index was 32 kg/m2. The mean duration of diabetes at baseline was 8 years and the mean baseline HbA1c was 7.0% with a mean baseline fasting plasma glucose of 142 mg/dL. At baseline, 12% of patients were treated with diet only, 40% were treated with one oral antidiabetic agent, 33% were treated with two oral antidiabetic agents, and 16% were treated with insulin alone or insulin in combination with an oral antidiabetic agent. At baseline, 76% of patients reported a history of hypercholesterolemia, 75% reported a history of hypertension, 11% reported a history of revascularization surgery, 10% reported a history of myocardial infarction, 10% reported a history of angina, and 5% reported a history of stroke. Forty-seven percent of the CYCLOSET-treated patients and 32% of the placebo-treated patients prematurely discontinued treatment. Adverse events leading to discontinuation of study drug occurred among 24% of the CYCLOSET-treated patients and 15% of the placebo-treated patients. This between-group difference was driven mostly by gastrointestinal adverse events, particularly nausea.
Table 1 summarizes the adverse events reported in ≥5% of patients treated with CYCLOSET in the Phase 3 clinical trials regardless of investigator assessment of causality. The most commonly reported adverse events (nausea, fatigue, vomiting, headache, dizziness) lasted a median of 14 days and were more likely to occur during the initial titration of CYCLOSET. None of the reports of nausea or vomiting were described as serious. There were no differences in the pattern of common adverse events across race groups or age groups (<65 years old vs. >65 years old). In the 52-week CYCLOSET safety trial, 11.5% of CYCLOSET-treated women compared to 3.6% of placebo-treated women reported vomiting. In this same trial, 5.4% of CYCLOSET-treated men compared to 2.8% of placebo-treated men reported vomiting.
| Monotherapy | CYCLOSET 1.6 mg – 4.8 mg N (%) | Placebo N (%) |
|---|---|---|
|
||
| N = 159 | N = 80 | N = 79 |
| Nausea | 26 (32.5) | 6 (7.6) |
| Rhinitis | 11 (13.8) | 3 (3.8) |
| Headache | 10 (12.5) | 7 (8.9) |
| Asthenia | 10 (12.5) | 5 (6.3) |
| Dizziness | 10 (12.5) | 6 (7.6) |
| Constipation | 9 (11.3) | 3 (3.8) |
| Sinusitis | 8 (10.0) | 2 (2.5) |
| Diarrhea | 7 (8.8) | 4 (5.1) |
| Amblyopia | 6 (7.5) | 1 (1.3) |
| Dyspepsia | 6 (7.5) | 2 (2.5) |
| Vomiting | 5 (6.3) | 1 (1.3) |
| Infection | 5 (6.3) | 4 (5.1) |
| Anorexia | 4 (5.0) | 1 (1.3) |
| Adjunct to Sulfonylurea (2 pooled 24-week studies) | ||
| N = 494 | N = 244 | N = 250 |
| Nausea | 62 (25.4) | 12 (4.8) |
| Asthenia | 46 (18.9) | 20 (8.0) |
| Headache | 41 (16.8) | 40 (16.0) |
| Flu syndrome | 23 (9.4) | 19 (7.6) |
| Constipation | 24 (9.8) | 11 (4.4) |
| Cold | 20 (8.2) | 20 (8.0) |
| Dizziness | 29 (11.9) | 14 (5.6) |
| Rhinitis | 26 (10.7) | 12 (4.8) |
| Sinusitis | 18 (7.4) | 16 (6.4) |
| Somnolence | 16 (6.6) | 5 (2.0) |
| Vomiting | 13 (5.3) | 8 (3.2) |
| Amblyopia | 13 (5.3) | 6 (2.4) |
| 52-Week Safety Trial† | ||
| N = 3070 | N = 2054 | N = 1016 |
| Nausea | 661 (32.2) | 77 (7.6) |
| Dizziness | 303 (14.8) | 93 (9.2) |
| Fatigue | 285 (13.9) | 68 (6.7) |
| Headache | 235 (11.4) | 84 (8.3) |
| Vomiting | 167 (8.1) | 32 (3.1) |
| Diarrhea | 167 (8.1) | 81 (8.0) |
| Constipation | 119 (5.8) | 52 (5.1) |
6.2 Postmarketing Experience
The active agent in CYCLOSET (bromocriptine mesylate) has been used in other formulations and often multiple times per day to treat hyperprolactinemia, acromegaly, and Parkinson's disease. The following adverse reactions have been identified during post approval use of bromocriptine mesylate for these indications, generally at doses higher than those approved for the treatment of type 2 diabetes. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7. Drug Interactions
- The active ingredient in CYCLOSET (bromocriptine mesylate) is highly bound to serum proteins. Therefore, CYCLOSET may increase the unbound fraction of other concomitantly used highly protein-bound therapies (e.g., salicylates, sulfonamides, chloramphenicol and probenecid), which may alter their effectiveness and risk for side effects.
- CYCLOSET is a dopamine receptor agonist. Concomitant use of dopamine receptor antagonists, such as neuroleptics (e.g., phenothiazines, butyrophenones, thioxanthenes), or metoclopramide may diminish the effectiveness of CYCLOSET, and CYCLOSET may diminish the effectiveness of these other therapies. The concurrent use of CYCLOSET with these agents has not been studied in clinical trials and is not recommended [see Warnings and Precautions (5.4)].
- CYCLOSET in combination with ergot-related drugs may cause an increase in the occurrence of ergot-related side effects, such as nausea, vomiting, and fatigue, and may also reduce the effectiveness of these ergot therapies when used to treat migraine. The concurrent use of these ergot agents within 6 hours of CYCLOSET dosing is not recommended.
- CYCLOSET is extensively metabolized by the liver via CYP3A4. Therefore, potent inhibitors or inducers of CYP3A4 may increase or reduce the circulating levels of CYCLOSET, respectively. Use caution when co-administering drugs that are inhibitors or inducers of CYP3A4. CYCLOSET dose should not exceed 1.6 mg once daily during concomitant use of a moderate CYP3A4 inhibitor (e.g., erythromycin). Concomitant use of strong CYP3A4 inhibitors (e.g., azole antimycotics, HIV protease inhibitors) with CYCLOSET should be avoided. Ensure adequate washout of the strong CYP3A4 inhibitor drug before initiating CYCLOSET treatment [see Clinical Pharmacology (12.3)].
- There are postmarketing reports of hypertension and tachycardia when bromocriptine was co-administered with sympathomimetic drugs (e.g., phenylpropanolamine and isometheptene) in postpartum women. There are limited clinical trial data supporting the safety of co-administering sympathomimetic drugs and CYCLOSET for more than 10 days. Therefore, concomitant use of these agents with CYCLOSET for more than 10 days duration is not recommended. Also, there are limited clinical trial data supporting the safety of selective 5-hydroxytryptamine1B (5-HT1B) agonists (e.g., sumatriptan) used concurrently with CYCLOSET, and the concomitant use of these agents with CYCLOSET should be avoided.
8. Use In Specific Populations
8.3 Nursing Mothers
CYCLOSET is contraindicated in women who are nursing their children. CYCLOSET contains bromocriptine which inhibits lactation. The indication for use of bromocriptine for inhibition of postpartum lactation was withdrawn based on postmarketing reports of stroke in this setting [see Contraindications (4), Adverse Reactions (6.2)].
8.4 Pediatric Use
The safety and effectiveness of CYCLOSET in pediatric patients have not been established.
8.5 Geriatric Use
In the two clinical trials of CYCLOSET add-on to sulfonylurea therapy and in the monotherapy trial, a total of 54 patients randomized to CYCLOSET were ≥65 years old. In the 52-week safety trial, 601 of the 2,054 CYCLOSET-treated patients (29%) were ≥65 years old. No overall differences in safety or effectiveness were observed between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. [See Clinical Studies (14).]
10. Overdosage
With another formulation of bromocriptine mesylate, the most commonly reported signs and symptoms associated with acute overdose were nausea, vomiting, constipation, diaphoresis, dizziness, pallor, severe hypotension, malaise, confusion, lethargy, drowsiness, delusions, hallucinations, and repetitive yawning. The lethal dose has not been established.
Treatment of overdose consists of removal of the drug by emesis (if conscious), gastric lavage, activated charcoal, or saline catharsis. Careful supervision and recording of fluid intake and output is essential. Hypotension should be treated by placing the patient in the Trendelenburg position and administering intravenous fluids. If satisfactory relief of hypotension cannot be achieved by using the above measures to their fullest extent, vasopressors should be considered.
11. Cycloset Description
CYCLOSET Tablets contain micronized bromocriptine mesylate, a dopamine receptor agonist. Bromocriptine mesylate is chemically designated [Ergotaman-3',6',18-trione, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-, monomethanesulfonate (salt), (5'α)-]. CYCLOSET is a single enantiomer with absolute configuration 5R, 8R, 2'R, 5'S, 11'S, 12'S.
The structural formula of bromocriptine is shown below:
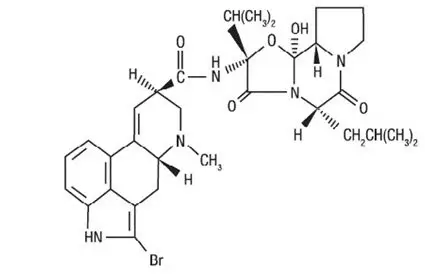
Bromocriptine mesylate in CYCLOSET is a white or slightly colored micronized crystalline powder with a molecular formula of C32H40BrN5O5∙CH4SO3 and a molecular weight of 750.72. CYCLOSET Tablets contain bromocriptine mesylate USP in an amount equivalent to 0.8 mg. of bromocriptine. Each tablet contains the following inactive ingredients: lactose, corn starch, magnesium stearate, colloidal silicon dioxide, and citric acid.
12. Cycloset - Clinical Pharmacology
12.1 Mechanism of Action
CYCLOSET contains bromocriptine mesylate, a sympatholytic, dopamine D2 receptor agonist. In patients with type 2 diabetes, timed morning administration of CYCLOSET is associated with increased insulin sensitivity and glucose disposal and reduced fasting and postprandial hyperglycemia throughout the meals of the day without raising plasma insulin levels.
14. Clinical Studies
A total of 3,723 patients with type 2 diabetes were randomized across 4 double-blind, placebo-controlled clinical trials conducted to evaluate the safety and glycemic efficacy of CYCLOSET. In the pooled 24-week monotherapy trial and the two 24-week add-on to sulfonylurea trials (N = 653), the mean age of the CYCLOSET-treated patients (N = 324) was 55 years, 71% were male and 73% Caucasian. In the 52-week safety trial (N = 3,070), the mean age for the entire study population was 60 years and 43% of patients were female, 68% were Caucasian, 17% were Black, 13% were Hispanic, and 1% were Asian.
In all 4 clinical trials, patients assigned to treatment with CYCLOSET received an initial dose of 0.8 mg, which was increased by 0.8 mg each week for 6 weeks (4.8 mg/day final dose) if no intolerance occurred or until the maximum tolerated dose ≥1.6 mg/day was reached. In patients with type 2 diabetes, treatment with CYCLOSET produced clinically significant improvements in HbA1c and postprandial glucose (PPG).
14.1 Monotherapy
A total of 159 overweight (body mass index ≥26.0 kg/m2 for males and ≥28.0 kg/m2 for females) adults with type 2 diabetes and inadequate glycemic control (HbA1c 7.5-11%) participated in a 24-week, placebo-controlled, monotherapy trial that evaluated the efficacy and safety of CYCLOSET as an adjunct to diet and exercise. Mean body weight at baseline was 93 kg in the CYCLOSET group and 96 kg in the placebo group. Mean HbA1c at baseline was 9.0% in the CYCLOSET group and 8.8% in the placebo group. Mean duration of diabetes at baseline was 5 years in the CYCLOSET group and 4 years in the placebo group. Of the 80 patients in the CYCLOSET group, 69% (N = 55) achieved the maximum daily dose of 4.8 mg. CYCLOSET improved HbA1c and fasting plasma glucose compared to placebo (Table 2). Mean change from baseline in body weight was +0.2 kg in the CYCLOSET group (N = 78) and +0.5 kg in the placebo group (N = 77).
| CYCLOSET N = 80 (1.6 - 4.8 mg) | Placebo N = 79 |
|
|---|---|---|
| P-value calculated by ANOVA; *p = 0.05, **p = 0.005 | ||
|
||
| HbA1c (%) | N = 74 | N = 74 |
| Baseline (mean) | 9.0 | 8.8 |
| Change from baseline (adj. mean) | -0.1 | 0.3 |
| Difference from placebo (adj. mean) | -0.4* | |
| Fasting Plasma Glucose (mg/dL) | N = 76 | N = 75 |
| Baseline (mean) | 215 | 205 |
| Change from baseline (adj. mean) | 0 | 23 |
| Difference from placebo (adj. mean) | -23** | |
14.3 Changes in Lipids and Blood Pressure
CYCLOSET does not have an unfavorable effect on fasting plasma lipids.
CYCLOSET has not demonstrated an unfavorable hypertensive effect on blood pressure. Hypotension has been reported with use of CYCLOSET in clinical trials [see Warnings and Precautions (5.1)].
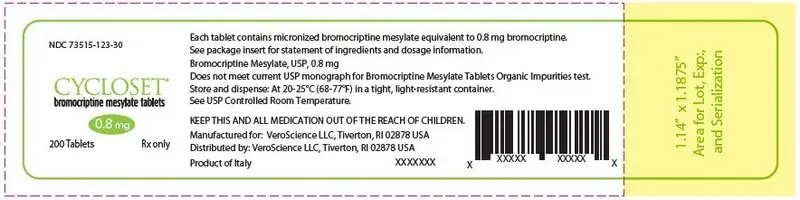
16. How is Cycloset supplied
CYCLOSET 0.8 mg tablets are WHITE and round with "C" on one side and "9" on the other.
The tablets are supplied as follows:
NDC 73515-123-30 unit-of-use bottles of 200
NDC 73515-123-21 unit-of-use bottles of 21 (samples only).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patients should be informed of the potential risks and benefits of CYCLOSET and of alternative therapies. Patients should also be informed about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and HbA1c testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and patients should be advised to seek medical advice promptly.
Patients should be advised that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, and diaphoresis. Hypotension and syncope may occur more frequently during initial therapy or with an increase in dose at any time. During early treatment with CYCLOSET, patients should be advised to make slow postural changes and to avoid situations that could predispose to serious injury if syncope was to occur.
Patients should be advised that CYCLOSET may cause somnolence. Advise patients not to operate heavy machinery if symptoms of somnolence occur.
Women who are nursing their children should be advised to not take CYCLOSET.
Physicians should instruct their patients to read the Patient Package Insert before starting CYCLOSET therapy and to reread it each time the prescription is renewed. Patients should be instructed to inform their healthcare provider if they develop any unusual symptoms or if any known symptom persists or worsens.
| CYCLOSET
bromocriptine mesylate tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - VEROSCIENCE LLC (556283799) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Pharmaceuticals Inc. | 005286822 | MANUFACTURE(73515-123) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AMRI Italy S.r.l. | 440365767 | API MANUFACTURE(73515-123) | |