Drug Detail:Danyelza (Naxitamab [ nax-it-a-mab ])
Drug Class: Miscellaneous antineoplastics
Highlights of Prescribing Information
DANYELZA® (naxitamab-gqgk) injection, for intravenous use
Initial U.S. Approval: 2020
WARNING: SERIOUS INFUSION-RELATED REACTIONS and NEUROTOXICITY
See full prescribing information for complete boxed warning
- Serious Infusion-Related Reactions: DANYELZA can cause serious infusion reactions, including cardiac arrest, anaphylaxis, hypotension, bronchospasm, and stridor. Premedicate prior to each DANYELZA infusion as recommended. Reduce the rate, interrupt infusion, or permanently discontinue DANYELZA based on severity (2.2, 2.3, 4, 5.1).
- Neurotoxicity: DANYELZA can cause severe neurotoxicity, including severe neuropathic pain, transverse myelitis, and reversible posterior leukoencephalopathy syndrome (RPLS). Premedicate to treat neuropathic pain as recommended. Permanently discontinue DANYELZA based on the adverse reaction and severity (2.2, 2.3, 5.2).
Indications and Usage for Danyelza
DANYELZA is a GD2-binding monoclonal antibody indicated, in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), for the treatment of pediatric patients 1 year of age and older and adult patients with relapsed or refractory high-risk neuroblastoma in the bone or bone marrow who have demonstrated a partial response, minor response, or stable disease to prior therapy.
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s). (1)
Danyelza Dosage and Administration
The recommended dosage of DANYELZA is 3 mg/kg/day (up to 150 mg/day), administered as an intravenous infusion after dilution on Days 1, 3, and 5 of each treatment cycle. Treatment cycles are repeated every 4 weeks until complete response or partial response, followed by 5 additional cycles every 4 weeks. Subsequent cycles may be repeated every 8 weeks.
Discontinue DANYELZA and GM-CSF for disease progression or unacceptable toxicity. Administer GM-CSF subcutaneously prior to and during each treatment cycle as recommended. (2.1)
Dosage Forms and Strengths
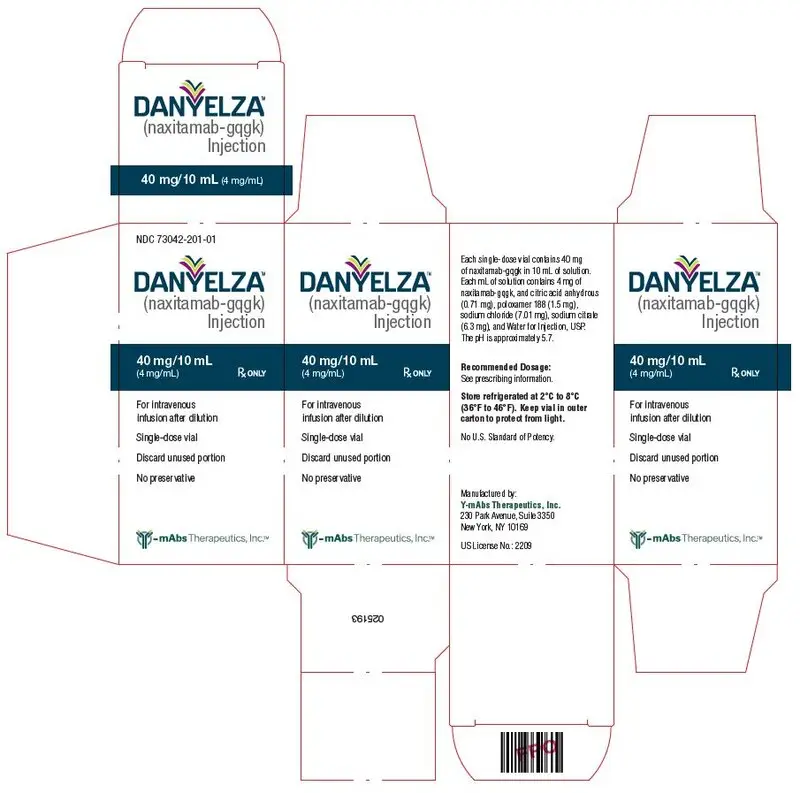
Injection: 40 mg/10 mL (4 mg/mL) in a single-dose vial. (3)
Contraindications
History of severe hypersensitivity reaction to naxitamab-gqgk. (4)
Warnings and Precautions
- Neurotoxicity: Peripheral neuropathy, neurological disorders of the eye, and prolonged urinary retention have also occurred. Permanently discontinue as recommended (2.3, 5.2)
- Hypertension: Monitor blood pressure during and after infusion as recommended. Withhold, reduce infusion rate, or discontinue based on severity. (5.3)
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of potential risk to a fetus and to use effective contraception (5.4)
Adverse Reactions/Side Effects
- The most common adverse reactions (≥25%) are infusion-related reaction, pain, tachycardia, vomiting, cough, nausea, diarrhea, decreased appetite, hypertension, fatigue, erythema multiforme, peripheral neuropathy, urticaria, pyrexia, headache, injection site reaction, edema, anxiety, localized edema, and irritability (6.1).
- The most common Grade 3 or 4 laboratory abnormalities (≥5%) are decreased lymphocytes, decreased neutrophils, decreased hemoglobin, decreased platelet count, decreased potassium, increased alanine aminotransferase, decreased glucose, decreased calcium, decreased albumin, decreased sodium, and decreased phosphate (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Y-mAbs Therapeutics, Inc, at 1-833-339-6227 (1-833-33YMABS), or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2020
Related/similar drugs
cisplatin, doxorubicin, vincristine, Adriamycin, dinutuximab, PlatinolFull Prescribing Information
1. Indications and Usage for Danyelza
DANYELZA is indicated, in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF), for the treatment of pediatric patients 1 year of age and older and adult patients with relapsed or refractory high-risk neuroblastoma in the bone or bone marrow who have demonstrated a partial response, minor response, or stable disease to prior therapy.
This indication is approved under accelerated approval based on overall response rate and duration of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
2. Danyelza Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of DANYELZA is 3 mg/kg/day (up to 150 mg/day) on Days 1, 3, and 5 of each treatment cycle, administered as an intravenous infusion after dilution [see Dosage and Administration (2.4 and 2.5)] in combination with GM-CSF subcutaneously as shown in Table 1. Refer to the GM-CSF Prescribing Information for recommended dosing information.
Treatment cycles are repeated every 4 weeks until complete response or partial response, followed by 5 additional cycles every 4 weeks. Subsequent cycles may be repeated every 8 weeks. Discontinue DANYELZA and GM-CSF for disease progression or unacceptable toxicity.
Administer pre-infusion medications and supportive treatment, as appropriate, during infusion. [see Dosage and Administration (2.2)]
The recommended dosage regimen for each treatment cycle is described below and in Table 1:
- Days -4 to 0: administer GM-CSF 250 µg/m2/day by subcutaneous injection, beginning 5 days prior to DANYELZA infusion.
- Days 1 to 5: administer GM-CSF 500 µg/m2/day by subcutaneous injection. Administer at least 1 hour prior to DANYELZA administration on Days 1, 3, and 5.
- Days, 1, 3, and 5: administer DANYELZA 3 mg/kg/day (up to 150 mg/day) by intravenous infusion.
| Day | -4 | -3 | -2 | -1 | 0 | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subcutaneous GM-CSF | 250 µg/m2/day | 500 µg/m2/day | |||||||||
| Intravenous DANYELZA | 3 mg/kg/day | 3 mg/kg/day | 3 mg/kg/day | ||||||||
2.3 Dosage Modifications for Adverse Reactions
The recommended dosage modifications for DANYELZA for adverse reactions are presented in Table 2.
| Adverse Reaction | Severity* | Dosage Modifications |
|---|---|---|
|
||
| Infusion-related reactions [see Warnings and Precautions (5.1)] | Grade 2 Defined as: Therapy or infusion interruption indicated but responds promptly to symptomatic treatment (e.g., antihistamines, NSAIDS, narcotics, IV fluids); prophylactic medications indicated for ≤24 hours |
|
| Grade 3 Defined as: Prolonged (e.g., not rapidly responsive to symptomatic medication and/or brief interruption of infusion); recurrence of symptoms following initial improvement; hospitalization indicated for clinical sequelae |
|
|
| Grade 4 infusion-related reactions Defined as: Life-threatening consequences: urgent intervention indicated or Grade 3 or 4 anaphylaxis |
|
|
| Pain [see Warnings and Precautions (5.2)] | Grade 3 unresponsive to maximum supportive measures |
|
| Reversible posterior leukoencephalopathy syndrome (RPLS) [see Warnings and Precautions (5.2)] | All Grades |
|
| Transverse myelitis [see Warnings and Precautions (5.2)] | All Grades |
|
| Peripheral neuropathy [see Warnings and Precautions (5.2)] | Motor neuropathy: Grade 2 or greater or Sensory neuropathy: Grade 3 or 4 |
|
| Neurological disorders of the eye [see Warnings and Precautions (5.2)] | Grade 2 to 4 resulting in decreased visual acuity or limiting activities of daily living |
|
| Subtotal or total vision loss |
|
|
| Prolonged urinary retention [see Warnings and Precautions (5.2)] | Persisting following discontinuation of opioids |
|
| Hypertension [see Warnings and Precautions (5.3)] | Grade 3 |
|
| Grade 4 |
|
|
| Other Adverse Reactions [see Adverse Reactions (6.1)] | Grade 3 |
|
| Grade 4 |
|
|
2.4 Preparation
- Use appropriate aseptic technique.
- Visually inspect vial for particulate matter and discoloration prior to administration. Discard vial if solution is discolored, cloudy, or contains particulate matter.
- Add appropriate quantities of 5% Albumin (Human), USP and 0.9% Sodium Chloride Injection, USP to an empty, sterile intravenous infusion bag large enough to hold the volume needed for the relevant dose as indicated in Table 3. Allow for 5-10 minutes of passive mixing.
- Withdraw the required dose of DANYELZA and inject into the infusion bag containing the 5% Albumin (Human), USP and 0.9% Sodium Chloride Injection, USP. Discard any unused portion of DANYELZA left in the vial.
Preparation instructions for DANYELZA are described in Table 3.
| DANYELZA dose (mg) | DANYELZA volume (mL) | Volume of 5% Albumin (Human), USP (mL) | Total infusion volume achieved by adding sufficient 0.9% Sodium Chloride Injection, USP (mL) | Final concentration of prepared DANYELZA infusion (mg/mL) |
|---|---|---|---|---|
| ≤ 80 | ≤ 20 | 10 | 50 | ≤ 1.6 |
| 81 to 120 | > 20 to 30 | 15 | 75 | 1.1 to 1.6 |
| 121 to 160 | > 30 to 40 | 20 | 100 | 1.2 to 1.6 |
| 161 to 200 | > 40 to 50 | 25 | 125 | 1.3 to 1.6 |
| 201 to 240 | > 50 to 60 | 30 | 150 | 1.3 to 1.6 |
| 241 to 280 | > 60 to 70 | 35 | 175 | 1.4 to 1.6 |
If not used immediately, store the diluted DANYELZA infusion solution at room temperature (15°C to 25°C [59ºF to 77ºF]) for up to 8 hours or refrigerate (2°C to 8°C [36°F to 46°F]) for up to 24 hours. Once removed from refrigeration, initiate infusion within 8 hours.
2.5 Administration
- Administer DANYELZA as a diluted intravenous infusion as recommended. Do not administer DANYELZA as an intravenous push or bolus [see Dosage and Administration (2.4)].
- For the first infusion (Cycle 1, Day 1), administer DANYELZA intravenously over 60 minutes.
For subsequent infusions, administer DANYELZA intravenously over 30 to 60 minutes, as tolerated. [see Dosage and Administration (2.1, 2.3)]. - Observe patients for a minimum of 2 hours following each infusion.
3. Dosage Forms and Strengths
Injection: 40 mg/10 mL (4 mg/mL) clear to slightly opalescent and colorless to slightly yellow solution in a single-dose vial.
4. Contraindications
DANYELZA is contraindicated in patients with a history of severe hypersensitivity reaction to naxitamab-gqgk. Reactions have included anaphylaxis [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Serious Infusion-Related Reactions
DANYELZA can cause serious infusion reactions requiring urgent intervention including fluid resuscitation, administration of bronchodilators and corticosteroids, intensive care unit admission, infusion rate reduction or interruption of DANYELZA infusion. Infusion-related reactions included hypotension, bronchospasm, hypoxia, and stridor [see Adverse Reactions (6.1)].
Serious infusion-related reactions occurred in 4% of patients in Study 201 and in 18% of patients in Study 12-230. Infusion-related reactions of any Grade occurred in 100% of patients in Study 201 and 94% of patients in Study 12-230. Hypotension of any grade occurred in 100% of patients in Study 201 and 89% of patients in Study 12-230.
In Study 201, 68% of patients experienced Grade 3 or 4 infusion reactions; and in Study 12-230, 32% of patients experienced Grade 3 or 4 infusion reactions. Anaphylaxis occurred in 12% of patients and 2 patients (8%) permanently discontinued DANYELZA due to anaphylaxis in Study 201. One patient in Study 12-230 (1.4%) experienced a Grade 4 cardiac arrest 1.5 hours following completion of DANYELZA infusion.
In Study 201, infusion reactions generally occurred within 24 hours of completing a DANYELZA infusion, most often within 30 minutes of initiation. Infusion reactions were most frequent during the first infusion of DANYELZA in each cycle. Eighty percent of patients required reduction in infusion rate and 80% of patients had an infusion interrupted for at least one infusion-related reaction.
Premedicate with an antihistamine, acetaminophen, an H2 antagonist and corticosteroid as recommended [see Dosage and Administration (2.2)]. Monitor patients closely for signs and symptoms of infusion reactions during and for at least 2 hours following completion of each DANYELZA infusion in a setting where cardiopulmonary resuscitation medication and equipment are available.
Reduce the rate, interrupt infusion, or permanently discontinue DANYELZA based on severity and institute appropriate medical management as needed [see Dosage and Administration (2.3) and Contraindications (4)].
5.2 Neurotoxicity
DANYELZA can cause severe neurotoxicity, including severe neuropathic pain, transverse myelitis, and reversible posterior leukoencephalopathy syndrome.
5.3 Hypertension
Hypertension occurred in 44% of patients in Study 201 and 28% of patients in Study 12-230 who received DANYELZA. Grade 3 or 4 hypertension occurred in 4% of patients in Study 201 and 7% of patients in Study 12-230. Four patients (6%) in Study 12-230 permanently discontinued DANYELZA due to hypertension. In both studies, most events occurred on the day of DANYELZA infusion and occurred up to 9 days following an infusion of DANYELZA.
Do not initiate DANYELZA in patients with uncontrolled hypertension. Monitor blood pressure during infusion, and at least daily on Days 1 to 8 of each cycle of DANYELZA and evaluate for complications of hypertension including RPLS [see Warnings and Precautions (5.2)]. Interrupt DANYELZA infusion and resume at a reduced rate, or permanently discontinue DANYELZA based on the severity [see Dosage and Administration (2.3) and Adverse Reactions (6.1)].
5.4 Embryo-Fetal Toxicity
Based on its mechanism of action, DANYELZA may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential, including pregnant women, of the potential risk to a fetus. Advise females of reproductive potential to use effective contraceptive during treatment with DANYELZA and for two months after the final dose. [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are also described elsewhere in the labeling:
- Serious Infusion-Related Reactions [see Warnings and Precautions (5.1)]
- Neurotoxicity [see Warnings and Precautions (5.2)]
- Hypertension [see Warnings and Precautions (5.3)]
6.1 Clinical TrialsExperience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of DANYELZA in combination with GM-CSF was evaluated in patients with refractory or relapsed high-risk neuroblastoma in bone or bone marrow who had demonstrated a partial response, minor response, or stable disease following initial or subsequent therapy, and in patients who were in second complete remission, from two open-label, single arm studies, Study 201 (n=25) and Study 12-230 (n=72). Patients received DANYELZA 9 mg/kg/cycle administered as three separate intravenous infusions of 3 mg/kg (Day 1, 3 and 5) in the first week of each cycle. Patients also received GM-CSF 250 µg/m2/day subcutaneously on Days -4 to 0 and GM-CSF 500 µg/m2/day subcutaneously on Days 1 to 5 [see Clinical Studies (14)].
The most common adverse reactions in Studies 201 and 12-230 (≥25% in either study) were infusion-related reaction, pain, tachycardia, vomiting, cough, nausea, diarrhea, decreased appetite, hypertension, fatigue, erythema multiforme, peripheral neuropathy, urticaria, pyrexia, headache, injection site reaction, edema, anxiety, localized edema and irritability. The most common Grade 3 or 4 laboratory abnormalities (≥5% in either study) were decreased lymphocytes, decreased neutrophils, decreased hemoglobin, decreased platelet count, decreased potassium, increased alanine aminotransferase, decreased glucose, decreased calcium, decreased albumin, decreased sodium and decreased phosphate.
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of anti-drug antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of anti-drug antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies or to other naxitamab products may be misleading.
In Study 201, 2 of 24 (8%) patients tested positive for anti-drug antibodies (ADA) after treatment with DANYELZA.
In Study 12-230, 27 of 117 patients (23%) tested positive for ADA after treatment with DANYELZA by an assay that was not fully validated; therefore, the incidence of ADA may not be reliable.
6.3 Postmarketing Experience/Spontaneous Reports
The following adverse reactions have been identified from expanded access reports with use of DANYELZA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Neurological: Transverse myelitis
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
DANYELZA may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of DANYELZA, in combination with GM-CSF for the treatment of relapsed or refractory high-risk neuroblastoma in the bone or bone marrow who have demonstrated a partial response, minor response or stable disease following prior therapy, have been established in pediatric patients 1 year of age and older.
Safety and effectiveness have not been established in pediatric patients younger than 1 year of age.
11. Danyelza Description
Naxitamab-gqgk is a glycolipid disialoganglioside (GD2)-binding recombinant humanized monoclonal IgG1 antibody, that contains human framework regions and murine complementarity-determining regions. Naxitamab-gqgk is produced in a Chinese hamster ovary cell line and has an approximate molecular weight of 144 kDa without glycosylation.
DANYELZA (naxitamab-gqgk) injection is a sterile, preservative-free, clear to slightly opalescent and colorless to slightly yellow solution for intravenous infusion. Each single-dose vial contains 40 mg of naxitamab-gqgk in 10 mL of solution. Each mL of solution contains 4 mg of naxitamab-gqgk, and citric acid anhydrous (0.71 mg), poloxamer 188 (1.5 mg), sodium chloride (7.01 mg), sodium citrate (6.3 mg), and Water for Injection, USP. The pH is approximately 5.7.
12. Danyelza - Clinical Pharmacology
12.1 Mechanism of Action
Naxitamab-gqgk binds to the glycolipid GD2. GD2 is a disialoganglioside that is overexpressed on neuroblastoma cells and other cells of neuroectodermal origin, including the central nervous system and peripheral nerves. In vitro, naxitamab-gqgk was able to bind to cell surface GD2 and induce complement dependent cytotoxicity (CDC) and antibody dependent cell-mediated cytotoxicity (ADCC).
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of naxitamab-gqgk have not been fully characterized.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been conducted to evaluate the carcinogenic or mutagenic potential of naxitamab-gqgk.
Dedicated studies evaluating the effects of naxitamab-gqgk on fertility in animals have not been conducted.
13.2 Animal Toxicology and/or Pharmacology
Non-clinical studies suggest that naxitamab-gqgk-induced neuropathic pain is mediated by binding of the antibody to the GD2 antigen located on the surface of peripheral nerve fibers and myelin and subsequent induction of immune-mediated cytotoxic activity.
In a nude rat model, slight-moderate hyperplasia and erosion of the glandular mucosa of the stomach occurred, occasionally accompanied by diffuse inflammation. Complete recovery of all histopathological findings in the stomachs of male rats was observed; however, only partial recovery was observed in the stomachs of female rats during the four week off-drug period.
14. Clinical Studies
The efficacy of DANYELZA in combination with GM-CSF was evaluated in two open-label, single arm trials in patients with high-risk neuroblastoma with refractory or relapsed disease in the bone or bone marrow, Study 201 and Study 12-230.
16. How is Danyelza supplied
DANYELZA (naxitamab-gqgk) injection is a sterile, preservative-free, clear to slightly opalescent and colorless to slightly yellow solution for intravenous infusion supplied as a carton containing one 40mg/10 mL (4 mg/mL) single-dose vial.
NDC 73042-201-01
17. Patient Counseling Information
Advise the patient and caregiver to read the FDA-approved patient labeling (Patient Information).
| This Patient Information has been approved by the U.S. Food and Drug Administration | Issued: 11/2020 | |||
| PATIENT INFORMATION
DANYELZA® (dan-YEL-zah) (naxitamab-gqgk) injection, for intravenous use |
||||
| What is the most important information I should know about DANYELZA? DANYELZA may cause serious side effects, including:
|
||||
|
| |||
|
||||
|
|
|||
|
||||
|
|
|||
|
||||
|
|
|||
|
||||
| What is DANYELZA?
DANYELZA is a prescription medicine used in combination with a medicine called granulocyte-macrophage colony-stimulating factor (GM-CSF) to treat children 1 year of age and older and adults with high-risk neuroblastoma in the bone or bone marrow that:
|
||||
| Do not receive DANYELZA if you have had a severe allergic reaction to naxitamab-gqgk, the active ingredient in DANYELZA. Ask your healthcare provider if you are not sure. | ||||
Before receiving DANYELZA, tell your healthcare provider about all your medical conditions, including if you:
|
||||
How will I receive DANYELZA?
|
||||
| What are the possible side effects of DANYELZA? DANYELZA may cause serious side effects, including:
|
||||
|
| |||
| The most common side effects of DANYELZA include: | ||||
|
|
|
||
| These are not all of the possible side effects of DANYELZA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
| General information about the safe and effective use of DANYELZA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about DANYELZA that is written for health professionals. |
||||
| What are the ingredients in DANYELZA? Active ingredient: naxitamab-gqgk Inactive ingredients: citric acid anhydrous, poloxamer 188, sodium chloride, sodium citrate, water for Injection; USP. Manufactured by: Y-mAbs Therapeutics, Inc., 230 Park Avenue, Suite 3350, New York, NY 10169 U.S. License number 2209 For more information, go to www.DANYELZA.com or call 1-833-339-6227 (1-833-33YMABS) |
||||
| DANYELZA
naxitamab injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Y-mAbs Therapeutics, Inc. (080671180) |