Drug Detail:Daurismo (Glasdegib [ glas-deg-ib ])
Drug Class: Hedgehog pathway inhibitors
Highlights of Prescribing Information
DAURISMO™ (glasdegib) tablets, for oral use
Initial U.S. Approval: 2018
WARNING: EMBRYO-FETAL TOXICITY
See full prescribing information for complete boxed warning.
- •
- DAURISMO can cause embryo-fetal death or severe birth defects when administered to a pregnant woman. DAURISMO is embryotoxic, fetotoxic, and teratogenic in animals. (5.1, 8.1)
- •
- Conduct pregnancy testing in females of reproductive potential prior to initiation of DAURISMO treatment. Advise females of reproductive potential to use effective contraception during treatment with DAURISMO and for at least 30 days after the last dose. (5.1, 8.1, 8.3)
- •
- Advise males of the potential risk of exposure through semen and to use condoms with a pregnant partner or a female partner of reproductive potential during treatment with DAURISMO and for at least 30 days after the last dose to avoid potential drug exposure. (5.1, 8.3)
Recent Major Changes
|
Dosage and Administration (2.2) |
3/2023 |
|
Warnings and Precautions (5.3) |
3/2023 |
Indications and Usage for Daurismo
DAURISMO is a hedgehog pathway inhibitor indicated, in combination with low-dose cytarabine, for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adult patients who are ≥75 years old or who have comorbidities that preclude use of intensive induction chemotherapy. (1)
Daurismo Dosage and Administration
Recommended dosage: 100 mg orally once daily. (2.1)
Dosage Forms and Strengths
Tablets: 100 mg, 25 mg. (3)
Contraindications
None. (4)
Warnings and Precautions
- •
- Blood Donation: Advise patients not to donate blood or blood products during treatment with DAURISMO and for at least 30 days after the last dose. (5.1)
- •
- QTc Interval Prolongation: Monitor electrocardiograms and electrolytes. If QTc prolongation occurs, interrupt treatment with DAURISMO. (2.2, 5.2)
- •
- Musculoskeletal Adverse Reactions: Obtain creatine phosphokinase (CPK) and serum creatinine levels prior to initiating DAURISMO and as indicated clinically thereafter. Temporary dose interruption, dose reduction, or discontinuation of DAURISMO may be required for musculoskeletal adverse reactions or serum CPK elevation. (2.2, 5.3)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥20%) are anemia, fatigue, hemorrhage, febrile neutropenia, musculoskeletal pain, nausea, edema, thrombocytopenia, dyspnea, decreased appetite, dysgeusia, mucositis, constipation, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- •
- Strong CYP3A4 Inhibitors: Consider alternative therapies that are not strong CYP3A4 inhibitors or monitor for increased risk of adverse reactions, including QTc interval prolongation. (7)
- •
- Strong CYP3A4 Inducers: Avoid concomitant use with DAURISMO. (7)
- •
- Moderate CYP3A4 Inducers: Avoid concomitant use with DAURISMO. If, concomitant use cannot be avoided, increase the dose of DAURISMO (2.3, 7).
- •
- QTc Prolonging Drugs: Avoid co-administration with DAURISMO. If co-administration is unavoidable, monitor for increased risk of QTc interval prolongation. (7)
Use In Specific Populations
Lactation: Advise women not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2023
Related/similar drugs
venetoclax, Venclexta, azacitidine, vincristine, cytarabine, ivosidenibFull Prescribing Information
WARNING: EMBRYO-FETAL TOXICITY
DAURISMO can cause embryo-fetal death or severe birth defects when administered to a pregnant woman. DAURISMO is embryotoxic, fetotoxic, and teratogenic in animals [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
Conduct pregnancy testing in females of reproductive potential prior to initiation of DAURISMO treatment. Advise females of reproductive potential to use effective contraception during treatment with DAURISMO and for at least 30 days after the last dose [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
Advise males of the potential risk of DAURISMO exposure through semen and to use condoms with a pregnant partner or a female partner of reproductive potential during treatment with DAURISMO and for at least 30 days after the last dose to avoid potential drug exposure [see Warnings and Precautions (5.1), Use in Specific Populations (8.3)].
1. Indications and Usage for Daurismo
DAURISMO is indicated, in combination with low-dose cytarabine, for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adult patients who are ≥75 years old or who have comorbidities that preclude use of intensive induction chemotherapy.
2. Daurismo Dosage and Administration
2.1 Recommended Dosage and Schedule
The recommended dosage of DAURISMO is 100 mg orally once daily on days 1 to 28 in combination with cytarabine 20 mg subcutaneously twice daily on days 1 to 10 of each 28-day cycle in the absence of unacceptable toxicity or loss of disease control. For patients without unacceptable toxicity, treat for a minimum of 6 cycles to allow time for clinical response.
Administer DAURISMO with or without food. Do not split or crush DAURISMO tablets. Administer DAURISMO about the same time each day. If a dose of DAURISMO is vomited, do not administer a replacement dose; wait until the next scheduled dose is due. If a dose of DAURISMO is missed or not taken at the usual time, administer the dose as soon as possible and at least 12 hours prior to the next scheduled dose. Return to the normal schedule the following day. Do not administer 2 doses of DAURISMO within 12 hours.
2.2 Monitoring and Dosage Modifications
Assess complete blood counts, electrolytes, renal, and hepatic function prior to the initiation of DAURISMO and at least once weekly for the first month. Monitor electrolytes and renal function once monthly for the duration of therapy. Obtain creatine phosphokinase (CPK) levels prior to initiating DAURISMO and as indicated clinically thereafter (e.g., if muscle symptoms are reported). Monitor electrocardiograms (ECGs) prior to the initiation of DAURISMO, approximately one week after initiation, and then once monthly for the next two months to assess for QTc prolongation. Repeat ECG if abnormal. Certain patients may require more frequent and ongoing ECG monitoring [see Warnings and Precautions (5.2)]. Manage any abnormalities promptly [see Adverse Reactions (6.1)].
See Table 1 for dosage modification guidelines for patients who develop an adverse reaction.
| Adverse Reaction | Recommended Action | |
|---|---|---|
| Abbreviations: CPK = creatine phosphokinase, ULN = upper limit of normal. | ||
|
||
|
QTc interval prolongation on at least 2 separate electrocardiograms (ECGs) [see Warnings and Precautions (5.2)] |
QTc interval greater than 480 ms to 500 ms |
Assess electrolyte levels and supplement as clinically indicated. |
|
QTc interval greater than 500 ms |
Assess electrolyte levels and supplement as clinically indicated. |
|
|
QTc interval prolongation with life-threatening arrhythmia |
Discontinue DAURISMO permanently. |
|
|
Musculoskeletal adverse reactions [see Warnings and Precautions (5.3)] |
Grade 3* or serum CPK elevation between 2.5 and 10 times upper limit of normal (ULN) |
Obtain CPK and serum creatinine levels at least weekly until resolution of clinical signs and symptoms. Interrupt DAURISMO until symptoms reduce to mild or return to baseline. Resume DAURISMO at the same dose level, or at a reduced dose of 50 mg. If toxicity recurs, discontinue DAURISMO. |
|
Grade 4* or serum CPK elevation greater than 10 times ULN |
Discontinue DAURISMO. |
|
|
Hematologic toxicity [see Adverse Reactions (6.1)] |
Platelets less than 10 Gi/L for more than 42 days in the absence of disease |
Discontinue DAURISMO and low-dose cytarabine permanently. |
|
Neutrophil count less than 0.5 Gi/L for more than 42 days in the absence of disease |
Discontinue DAURISMO and low-dose cytarabine permanently. |
|
|
Nonhematologic toxicity [see Adverse Reactions (6.1)] |
Grade 3* |
Interrupt DAURISMO and/or low-dose cytarabine until symptoms reduce to mild or return to baseline. |
|
Grade 4* |
Discontinue DAURISMO and low-dose cytarabine permanently. |
|
2.3 Dosage Modification for Concomitant Use with Moderate CYP3A4 Inducers
Avoid concomitant use of DAURISMO with moderate CYP3A4 inducers. If concomitant use of moderate CYP3A4 inducers cannot be avoided, increase the DAURISMO dosage as tolerated as shown in Table 2. After the moderate CYP3A4 inducer has been discontinued for 7 days, resume the DAURISMO dose taken prior to initiating the moderate CYP3A4 inducer [see Drug Interactions (7), Clinical Pharmacology (12.3)].
| Current Dosage | Adjusted Dosage |
|---|---|
|
100 mg orally once daily |
200 mg orally once daily |
|
50 mg orally once daily |
100 mg orally once daily |
3. Dosage Forms and Strengths
DAURISMO 100 mg tablets: round, pale orange film-coated tablet debossed with "Pfizer" on one side and "GLS 100" on the other.
DAURISMO 25 mg tablets: round, yellow film-coated tablet debossed with "Pfizer" on one side and "GLS 25" on the other.
5. Warnings and Precautions
5.1 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal embryo-fetal developmental toxicity studies, DAURISMO can cause embryo-fetal death or severe birth defects when administered to a pregnant woman. There are no clinical data on the use of DAURISMO in pregnant women. In animal embryo-fetal developmental toxicity studies, glasdegib caused embryotoxicity, fetotoxicity and teratogenicity at maternal exposures that were less than the human exposure at the recommended human dose of 100 mg [see Use in Specific Populations (8.1, 8.2), Clinical Pharmacology (12.1)]. Advise pregnant women of the potential risk to the fetus.
Females of Reproductive Potential
DAURISMO is not recommended for use during pregnancy. Conduct pregnancy testing in female patients of reproductive potential prior to initiating DAURISMO treatment. Advise females of reproductive potential to use effective contraception during treatment with DAURISMO and for at least 30 days after the last dose. Advise women not to breastfeed during treatment with DAURISMO and for at least 30 days after the last dose [see Use in Specific Populations (8.2, 8.3)].
Males
Advise male patients with female partners of the potential risk of exposure through semen and to use effective contraception, including a condom, even after vasectomy, to avoid drug exposure to a pregnant partner or a female partner of reproductive potential during treatment with DAURISMO and for at least 30 days after the last dose [see Use in Specific Populations (8.3)].
5.2 QTc Interval Prolongation
Patients treated with DAURISMO can develop QTc prolongation and ventricular arrhythmias, including ventricular fibrillation and ventricular tachycardia. Of the 98 evaluable patients treated with DAURISMO 100 mg in combination with low-dose cytarabine in the clinical trial, 5% were found to have a QTc interval greater than 500 ms and 4% of patients had an increase from baseline QTc greater than 60 ms. The clinical trial excluded patients with baseline QTc of greater than 470 ms or with a history of long QT syndrome or uncontrolled cardiovascular disease.
Monitor electrocardiograms (ECGs) and electrolytes [see Dosage and Administration (2.2)]. Concomitant use of DAURISMO with drugs known to prolong the QTc interval and CYP3A4 inhibitors may increase the risk of QTc interval prolongation [see Drug Interactions (7), Clinical Pharmacology (12.2)]. In patients with congenital long QT syndrome, congestive heart failure, electrolyte abnormalities, or those who are taking medications known to prolong the QTc interval, more frequent ECG monitoring is recommended.
Interrupt DAURISMO if QTc increases to greater than 500 ms. Discontinue DAURISMO permanently for patients who develop QTc interval prolongation with signs or symptoms of life-threatening arrhythmia [see Dosage and Administration (2.2)].
5.3 Musculoskeletal Adverse Reactions
Musculoskeletal adverse reactions, which may be accompanied by CPK elevations, have occurred with DAURISMO and other drugs which inhibit the hedgehog (Hh) pathway. In BRIGHT AML 1003, musculoskeletal adverse reactions occurred in 45% of patients treated, with 2% (7/79) reported as Grade 3 or higher. The most frequent manifestations of musculoskeletal adverse reactions reported were musculoskeletal pain (30%) and muscle spasms (15%). Increased CPK laboratory values occurred in 16% of patients [see Adverse Reactions (6.1)].
Obtain baseline CPK levels prior to initiating DAURISMO and as clinically indicated (e.g., if muscle symptoms are reported). Obtain CPK and serum creatinine levels at least weekly in patients with musculoskeletal adverse reactions with concurrent CPK elevation greater than 2.5 times ULN until resolution of clinical signs and symptoms. Depending on the severity of symptoms, temporary dose interruption, dose reduction, or discontinuation of DAURISMO may be required for musculoskeletal adverse reactions or serum CPK elevation [see Dosage and Administration (2.2)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- QTc Interval Prolongation [see Warnings and Precautions (5.2)]
- •
- Musculoskeletal Adverse Reactions [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety profile of DAURISMO is based on experience in the BRIGHT AML 1003 study for 111 adults with newly-diagnosed AML and 14 adults with other conditions for which DAURISMO is not indicated [see Clinical Studies (14)]. Patients were treated with DAURISMO 100 mg daily in combination with low-dose cytarabine (N=84) or low-dose cytarabine alone (N=41). The median duration of treatment in the DAURISMO with low-dose cytarabine arm was 83 days (range 3 to 972 days), and the median duration of treatment in the low-dose cytarabine alone arm was 47 days (range 6 to 239 days). The median exposure to DAURISMO in the DAURISMO with low-dose cytarabine arm was 76 days (range 3 to 954 days). Thirty-two patients (38%) were treated with DAURISMO with low-dose cytarabine for at least 6 months and 14 patients (17%) were treated for at least 1 year.
Serious adverse reactions were reported in 79% of patients treated in the DAURISMO with low-dose cytarabine arm. The most common (≥5%) serious adverse reactions in patients receiving DAURISMO with low-dose cytarabine were febrile neutropenia (29%), pneumonia (23%), hemorrhage (12%), anemia (7%), and sepsis (7%).
Dose reductions associated with adverse reactions were reported in 26% of patients treated with DAURISMO with low-dose cytarabine, and the most common reasons (≥2%) for dose reductions due to adverse reactions were muscle spasms (5%), fatigue (4%), febrile neutropenia (4%), anemia (2%), thrombocytopenia (2%), and ECG QT prolonged (2%). Adverse reactions leading to permanent discontinuation were reported in 36% of patients treated with DAURISMO with low-dose cytarabine, and the most common (≥2%) reasons for permanent discontinuation were pneumonia (6%), febrile neutropenia (4%), sepsis (4%), sudden death (2%), myocardial infarction (2%), nausea (2%), and renal insufficiency (2%).
Adverse reactions reported in the first 90 days of therapy on the BRIGHT AML 1003 study are shown in Table 3.
| Body System | Adverse Reactions | DAURISMO With Low-Dose Cytarabine
N=84 | Low-Dose Cytarabine
N=41 |
||
|---|---|---|---|---|---|
| All Grades
% | Grade ≥ 3
% | All Grades
% | Grade ≥ 3
% |
||
| Abbreviations: N = number of patients. Preferred terms were retrieved by applying the Medical Dictionary for Regulatory Activities (MedDRA) version 19.1. BRIGHT AML 1003 used National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Adverse reactions include events that commenced within 28 days after the last treatment dose. |
|||||
|
|||||
|
Blood and lymphatic system disorder |
Anemia |
43 |
41 |
42 |
37 |
|
Hemorrhage‡ |
36 |
6 |
42 |
12 |
|
|
Febrile neutropenia |
31 |
31 |
22 |
22 |
|
|
Thrombocytopenia |
30 |
30 |
27 |
24 |
|
|
General disorders and administration site conditions |
Fatigue§ |
36 |
14 |
32 |
7 |
|
Edema¶ |
30 |
0 |
20 |
2 |
|
|
Mucositis# |
21 |
1 |
12 |
0 |
|
|
Pyrexia |
18 |
1 |
22 |
2 |
|
|
Chest painÞ |
12 |
1 |
2 |
0 |
|
|
Musculoskeletal and connective tissue disorders |
Musculoskeletal painß |
30 |
2 |
17 |
2 |
|
Muscle spasmà |
15 |
0 |
5 |
0 |
|
|
Gastrointestinal disorders |
Nausea |
29 |
1 |
12 |
2 |
|
Constipation |
20 |
1 |
12 |
0 |
|
|
Abdominal painè |
19 |
0 |
12 |
0 |
|
|
Diarrheað |
18 |
4 |
22 |
0 |
|
|
Vomiting |
18 |
2 |
10 |
2 |
|
|
Respiratory thoracic and mediastinal disorders |
Dyspneaø |
23 |
11 |
24 |
7 |
|
Coughý |
18 |
0 |
15 |
2 |
|
|
Metabolism and nutrition disorders |
Decrease appetite |
21 |
1 |
7 |
2 |
|
Nervous system disorders |
Dysgeusia£ |
21 |
0 |
2 |
0 |
|
Dizziness |
18 |
1 |
7 |
0 |
|
|
Headache |
12 |
0 |
10 |
2 |
|
|
Skin and subcutaneous tissue disorders |
Rash¥ |
20 |
2 |
7 |
2 |
|
Infection and infestations |
PneumoniaΠ|
19 |
15 |
24 |
22 |
|
Investigations |
Hyponatremia |
11 |
6 |
0 |
0 |
|
Platelet count decreased |
15 |
15 |
10 |
10 |
|
|
Weight decreased |
13 |
0 |
2 |
0 |
|
|
White blood cell count decreased |
11 |
11 |
5 |
2 |
|
|
Cardiac disorders |
Atrial arrhythmiaœ |
13 |
4 |
7 |
2 |
|
Renal and urinary disorders |
Renal insufficiencyƉ |
19 |
5 |
10 |
0 |
The adverse reactions muscle spasms (4 in 12 patients) and decreased appetite (2 in 10 patients) worsened (i.e. progressed from Grades ≤ 2 to Grade 3 or higher) after the first 90 days of therapy in BRIGHT AML 1003.
Additional clinically-significant adverse reactions occurring in < 10% of patients treated with DAURISMO and low-dose cytarabine in BRIGHT AML 1003 include:
- •
- Dental disorders: loose tooth and toothache
- •
- Skin and subcutaneous tissue disorders: alopecia
- •
- Cardiac disorders: QT interval prolonged
Changes in selected post-baseline laboratory values that were observed in patients with newly-diagnosed AML and other conditions for which DAURISMO is not indicated in the clinical trial are shown in Table 4.
| DAURISMO with Low-Dose Cytarabine | Low-Dose Cytarabine | |||||
|---|---|---|---|---|---|---|
| Laboratory Abnormality | N | All Grades
% | Grade 3 or 4†
% | N | All Grades
% | Grade 3 or 4†
% |
| Abbreviations: N = number of patients; AST = aspartate aminotransferase; ALT = alanine aminotransferase; CPK = creatinine phosphokinase. BRIGHT AML 1003 used National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. |
||||||
|
||||||
|
Creatinine increased |
81 |
96 |
1 |
40 |
80 |
5 |
|
Hyponatremia |
81 |
54 |
7 |
39 |
41 |
8 |
|
Hypomagnesemia |
81 |
33 |
0 |
39 |
23 |
0 |
|
AST increased |
80 |
28 |
1 |
40 |
23 |
0 |
|
Blood bilirubin increased |
80 |
25 |
4 |
39 |
33 |
3 |
|
ALT increased |
80 |
24 |
0 |
40 |
28 |
3 |
|
Alkaline phosphatase increased |
80 |
23 |
0 |
40 |
28 |
3 |
|
Hyperkalemia |
81 |
16 |
1 |
40 |
8 |
3 |
|
CPK increased |
38 |
16 |
0 |
17 |
6 |
0 |
|
Hypokalemia |
81 |
15 |
0 |
40 |
23 |
0 |
The following laboratory abnormalities worsened (i.e. progressed from Grades ≤ 2 to Grade 3 or higher) after the first 90 days of therapy in BRIGHT AML 1003:
- •
- hypophosphatemia (8 in 38 patients), creatinine increased (2 in 39 patients), and ALT increased (2 in 40 patients).
7. Drug Interactions
|
Strong CYP3A Inhibitors |
|
|
Clinical Impact |
|
|
Prevention or Management |
|
|
Strong and Moderate CYP3A Inducers |
|
|
Clinical Impact |
Co-administration of DAURISMO with strong and moderate CYP3A inducers decreased glasdegib plasma concentrations [see Clinical Pharmacology (12.3)].
|
|
Prevention or Management |
|
|
QTc Prolonging Drugs |
|
|
Clinical Impact |
Co-administration of DAURISMO with QTc prolonging drugs may increase the risk of QTc interval prolongation [see Warnings and Precautions (5.2)]. |
|
Prevention or Management |
|
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on its mechanism of action and findings in animal embryo-fetal developmental toxicity studies, DAURISMO can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no clinical data on the use of DAURISMO in pregnant women to inform of a drug-associated risk of major birth defects and miscarriage. DAURISMO is not recommended for use during pregnancy. Conduct pregnancy testing in female patients of reproductive potential prior to initiating treatment with DAURISMO. Report pregnancy exposures to Pfizer at 1-800-438-1985.
In animal embryo-fetal developmental toxicity studies, repeat-dose oral administration of DAURISMO during organogenesis at maternal exposures that were less than the human exposure at the recommended dose resulted in embryotoxicity, fetotoxicity and teratogenicity in rats and rabbits (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
In embryo-fetal developmental toxicity studies, glasdegib was orally administered to pregnant rats and rabbits at doses up to 100 mg/kg/day during the period of organogenesis. Glasdegib resulted in embryo-fetal lethality (e.g., increased postimplantation loss and decreased numbers of live fetuses) in rats and rabbits at 50 mg/kg/day and 5 mg/kg/day, respectively, at maternal exposures approximately 4-times and 3-times the human exposure at the recommended dose [based on Cmax (rat) and AUC (rabbit)]. Doses of ≥ 10 mg/kg in rat [approximately 0.6-times the human exposure (Cmax) at the recommended dose] and ≥ 5 mg/kg in rabbit resulted in fetal developmental abnormalities and malformations consisting of craniofacial malformations, malformed limbs, paws/digits, trunk and tail, dilation of brain, malpositioned/malformed eyes, misshapen head, small tongue, absent palate, teeth and viscera, diaphragmatic hernia, edema, heart defects, rib and vertebral abnormalities, malformed or absent structures in the appendicular skeleton.
8.2 Lactation
Risk Summary
There are no data on the presence of glasdegib or its active metabolites in human milk, the effects of the drug on the breastfed child, or its effect on milk production. Because of the potential for serious adverse reactions in a breastfed child from DAURISMO, advise women who are taking DAURISMO not to breastfeed or provide breast milk to infants or children during treatment with DAURISMO and for at least 30 days after the last dose.
8.3 Females and Males of Reproductive Potential
DAURISMO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Conduct pregnancy testing in females of reproductive potential within 7 days prior to initiating therapy with DAURISMO.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with DAURISMO and at least 30 days after the last dose.
Males
It is not known if glasdegib is present in semen. Advise males of the potential risk of exposure through semen and to use effective contraception, including a condom, even after a vasectomy, to avoid drug exposure to a pregnant partner or a female partner of reproductive potential during treatment with DAURISMO and for at least 30 days after the last dose. Advise males to not donate semen during treatment with DAURISMO for at least 30 days after the last dose [see Nonclinical Toxicology (13.1)].
Infertility
Males
Based on findings in repeat-dose animal toxicity studies in rats, DAURISMO may impair fertility in males of reproductive potential. Some effects on male reproductive organs did not recover [see Nonclinical Toxicology (13.1)]. Men should seek advice on effective fertility preservation before treatment.
8.4 Pediatric Use
The safety and effectiveness of DAURISMO have not been established in pediatric patients. In repeat-dose toxicity studies in rats, oral administration of DAURISMO resulted in adverse changes in growing bone, teeth, and testis. Effects on bone consisted of partial to complete closure of the epiphyseal plate. Effects in growing incisor teeth included degeneration/necrosis of ameloblasts, and complete tooth loss with oral ulceration. Reproductive tissue toxicity was evidenced by testicular degeneration and hypospermatogenesis. These effects in bone, teeth and testis were observed after administration of DAURISMO for 26 weeks at greater than or equal to 50 mg/kg/day corresponding to approximately 6.6-times the steady state AUC in patients at the recommended human dose.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of DAURISMO with low-dose cytarabine (N=88), 98% of the patients were age 65 years or older and 60% of the patients were age 75 years or older. There were insufficient patients younger than age 65 years to determine differences in adverse reactions reported from patients older than 65.
8.6 Renal Impairment
No dosage modification is recommended for patients with mild to severe renal impairment (estimated glomerular filtration rate [eGFR] 15 to 89 mL/min). Monitor patients with severe renal impairment (eGFR 15 to 29 mL/min) for increased risk of adverse reactions, including QTc interval prolongation, due to increased glasdegib concentrations [see Clinical Pharmacology (12.3)].
10. Overdosage
There is no specific antidote for DAURISMO. Management of DAURISMO overdose should include symptomatic treatment and ECG monitoring.
Glasdegib has been administered in clinical studies up to a dose of 640 mg/day. At the highest dosage, the adverse reactions that were dose limiting were nausea, vomiting, dehydration, hypotension, fatigue, and dizziness.
11. Daurismo Description
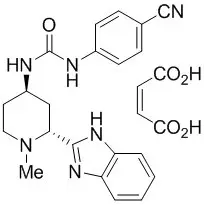
DAURISMO (glasdegib) is a hedgehog pathway inhibitor. It is formulated with the maleate salt of glasdegib. The molecular formula for glasdegib maleate is C25H26N6O5. The molecular weight for glasdegib maleate is 490.51 Daltons. The chemical name of glasdegib maleate is 1-((2R,4R)-2-(1H-benzo[d]imidazol-2-yl)-1-methylpiperidin-4-yl)-3-(4-cyanophenyl)urea maleate. The molecular structure is shown below:
Glasdegib maleate is a white to pale colored powder with pKa values of 1.7 and 6.1. The aqueous solubility of glasdegib maleate is 1.7 mg/mL.
DAURISMO (glasdegib) is supplied as a film-coated tablet for oral use containing either 100 mg glasdegib (equivalent to 131.1 mg glasdegib maleate) or 25 mg of glasdegib (equivalent to 32.8 mg glasdegib maleate) together with microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, and magnesium stearate as inactive ingredients in the tablet. The film-coating consists of Opadry II® Beige (33G170003) and Opadry II® Yellow (33G120011) containing: hypromellose, titanium dioxide, lactose monohydrate, macrogol, triacetin, iron oxide yellow, and iron oxide red.
12. Daurismo - Clinical Pharmacology
12.1 Mechanism of Action
Glasdegib is an inhibitor of the Hedgehog pathway. Glasdegib binds to and inhibits Smoothened, a transmembrane protein involved in hedgehog signal transduction.
In a murine xenotransplant model of human AML, glasdegib in combination with low-dose cytarabine, inhibited increases in tumor size and reduced the percentage of CD45+/CD33+ blasts in the marrow to a greater extent than glasdegib or low-dose cytarabine alone.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of glasdegib administration on corrected QT interval (QTc) was evaluated in a randomized, single-dose, double-blind, 4-way crossover, placebo- and open-label moxifloxacin-controlled study in 36 healthy subjects. At therapeutic plasma concentrations for the recommended dose, achieved with a single dose of 150 mg DAURISMO, the largest placebo and baseline-adjusted QTc interval change was 8 ms (90% CI: 6, 10 ms). At a two-fold therapeutic plasma concentration, achieved with a single dose of 300 mg DAURISMO, the QTc change was 13 ms (90% CI: 11, 16 ms). Glasdegib is associated with concentration-dependent QTc prolongation.
12.3 Pharmacokinetics
DAURISMO at 5 mg to 600 mg once daily (0.05 to 6 times the recommended dose) result in a dose proportional increase in glasdegib peak concentrations (Cmax) and area under the curve over the dosing interval (AUC0-Tau). Steady-state plasma levels are reached by 8 days of daily dosing. The median accumulation ratio of glasdegib ranged from 1.2 to 2.5 following once-daily dosing.
At DAURISMO 100 mg once daily, the geometric mean (geometric coefficient of variation, % CV) of glasdegib Cmax was 1252 ng/mL (44%) and AUC0-Tau was 17210 ng*hr/mL (54%) in patients with cancer.
Absorption
The mean absolute bioavailability of DAURISMO is 77%. Following 100 mg once daily dosing, glasdegib median time to peak concentrations (Tmax) at steady-state ranged from 1.3 hours to 1.8 hours.
Distribution
Glasdegib is 91% bound to human plasma proteins in vitro. The geometric mean (%CV) apparent volume of distribution (Vz/F) was 188 L (20%) in patients with hematologic malignancies.
Elimination
Glasdegib has a mean (± SD) half-life of 17.4 h (3.7) and geometric mean (%CV) apparent clearance of 6.45 L/h (25%) following 100 mg once daily dosing in patients with hematologic malignancies.
Specific Populations
Age (25 to 92 years), sex, race (White, Black, Asian), body weight (43.5 to 145.6 kg), mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin 1–1.5 × ULN and any AST), and mild renal impairment (creatinine clearance 60–89 mL/min) did not have clinically meaningful effects on the pharmacokinetics of glasdegib.
Patients with Renal Impairment
Following administration of a single dose of DAURISMO 100 mg, glasdegib AUC0-INF increased by 2.1-fold in subjects with moderate (eGFR 30 to 59 mL/min) and severe (eGFR 15 to 29 mL/min) renal impairment compared to subjects with normal renal function (eGFR ≥90 mL/min). The pharmacokinetics of glasdegib have not been studied in patients with end stage renal disease requiring hemodialysis.
Patients with Hepatic Impairment
Following administration of a single dose of DAURISMO 100 mg, glasdegib AUC0-INF increased by 11% in subjects with moderate hepatic impairment (Child-Pugh B) and decreased by 24% in subjects with severe hepatic impairment (Child-Pugh C) compared to subjects with normal hepatic function.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of Strong CYP3A4 Inhibitors on Glasdegib: Co-administration of ketoconazole (a strong inhibitor of CYP3A4) with DAURISMO increased the glasdegib AUC0-INF by 2.4-fold and Cmax by 1.4-fold over glasdegib given alone [see Drug Interactions (7)].
Effect of Strong and Moderate CYP3A4 Inducers on Glasdegib: Co-administration of rifampin (a strong inducer of CYP3A4) with DAURISMO decreased glasdegib AUC0-INF by 70% and Cmax by 35% [see Drug Interactions (7)]. Co-administration of efavirenz (moderate CYP3A4 inducer) is predicted to decrease glasdegib AUC0-INF by 55% and Cmax by 25%.
In Vitro Studies
Effect of Glasdegib on Cytochrome P450 (CYP) Substrates: Glasdegib does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A, and does not induce CYP1A2, CYP2B6, and CYP3A in vitro.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with glasdegib.
Glasdegib was not mutagenic in vitro in the bacterial reverse mutation (Ames) assay and was not clastogenic in the in vitro chromosome aberration assay in human lymphocytes. Glasdegib was not clastogenic or aneugenic in the rat micronucleus assay.
Based on nonclinical safety findings, glasdegib has the potential to impair reproductive function in males. Men should seek advice on effective fertility preservation before treatment. In repeat-dose toxicity studies in rats, findings observed in the male reproductive tract included adverse testicular changes with glasdegib at doses ≥50 mg/kg/day, and consisted of minimal to severe hypospermatogenesis characterized by partial to complete loss of spermatogonia, spermatocytes and spermatids and testicular degeneration. Hypospermatogenesis did not recover whereas testicular degeneration did recover. The dose at which testicular effects were observed in male rats was identified as 50 mg/kg/day with corresponding systemic exposures that were approximately 6.6-times (based on AUC) those associated with the observed human exposure at the 100 mg once daily dose.
14. Clinical Studies
The efficacy of DAURISMO in combination with low-dose cytarabine was evaluated in a multicenter, open-label, randomized study (Study BRIGHT AML 1003, NCT01546038) that included 115 patients age 55 years or older with newly-diagnosed AML who met at least one of the following criteria: a) age ≥75 years, b) severe cardiac disease, c) baseline Eastern Cooperative Oncology Group (ECOG) performance status of 2, or d) baseline serum creatinine >1.3 mg/dL. Patients were randomized 2:1 to receive DAURISMO at a 100 mg daily dose with low-dose cytarabine 20 mg subcutaneously twice daily on days 1 to 10 of a 28-day cycle (N=77) or low-dose cytarabine alone (N=38) in 28-day cycles until disease progression or unacceptable toxicity. Patients were stratified by cytogenetic risk (good/intermediate or poor).
The baseline demographic and disease characteristics are shown in Table 6. The two treatment arms were generally balanced with respect to the baseline demographics and disease characteristics (see Table 6).
| Demographic and Disease Characteristics | DAURISMO With Low-Dose Cytarabine
(N=77) | Low-Dose Cytarabine Alone
(N=38) |
|---|---|---|
| Abbreviations: AML = acute myeloid leukemia; N = number of patients; ECOG PS = Eastern Cooperative Oncology Group Performance Status. | ||
|
||
|
Demographics | ||
|
Age | ||
|
Median (Min, Max) (Years) |
77 (64, 92) |
76 (58, 83) |
|
≥ 75 years N (%) |
47 (61) |
23 (61) |
|
Sex, N (%) | ||
|
Male |
59 (77) |
23 (61) |
|
Female |
18 (23) |
15 (39) |
|
Race, N (%) | ||
|
White |
75 (97) |
38 (100) |
|
Black or African American |
1 (1) |
0 (0) |
|
Asian |
1 (1) |
0 (0) |
|
Disease History, N (%) | ||
|
De Novo AML |
38 (49) |
18 (47) |
|
Secondary AML |
39 (51) |
20 (53) |
|
Prior Hypomethylating Agent Use |
11 (14) |
6 (16) |
|
ECOG PS*, N (%) | ||
|
0 to 1 |
35 (46) |
20 (53) |
|
2 |
41 (53) |
18 (47) |
|
Cytogenetic Risk Status, N (%) | ||
|
Good/Intermediate |
48 (62) |
21 (55) |
|
Poor |
29 (38) |
17 (45) |
|
Baseline Severe Cardiac Disease |
51 (66) |
20 (53) |
|
Baseline Serum Creatinine >1.3 mg/dL |
15 (19) |
5 (13) |
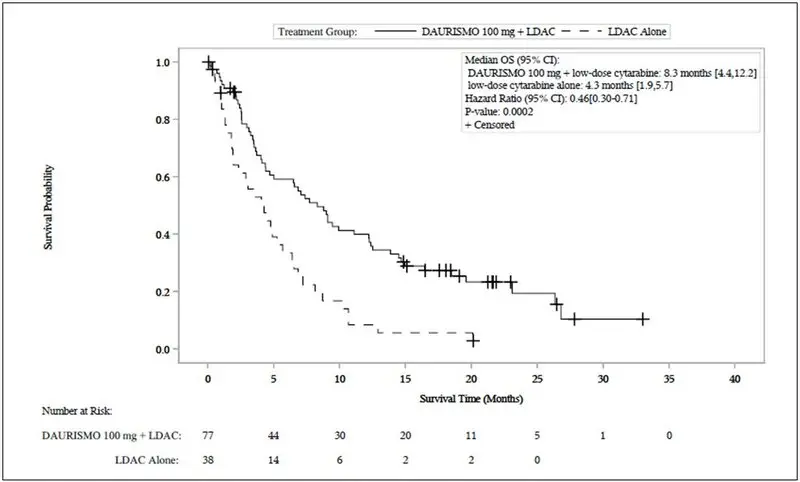
Efficacy was established on the basis of overall survival (OS) from the date of randomization to death from any cause. With a median follow-up of approximately 20 months, the DAURISMO with low-dose cytarabine arm was superior to low-dose cytarabine alone arm (Figure 1). The efficacy results are shown in Table 7. Improvement in OS was consistent across prespecified cytogenetic risk subgroups.
| Endpoint/Study Population | DAURISMO With Low-Dose Cytarabine | Low-Dose Cytarabine Alone |
|---|---|---|
| Abbreviations: AML = acute myeloid leukemia; N = number of patients; OS = overall survival; CI = confidence interval; CR = complete response. | ||
|
||
|
OS |
N=77 |
N=38 |
|
Median survival, months (95% CI) |
8.3 (4.4, 12.2) |
4.3 (1.9, 5.7) |
|
Hazard ratio (95% CI)* |
0.46 (0.30, 0.71) |
|
|
p-value† |
0.0002 |
|
|
CR |
N=14 |
N=1 |
|
CR rate (in %, 95% CI) |
18.2 (10.3, 28.6) |
2.6 (0.1, 13.8) |
Figure 1. BRIGHT AML 1003 – Kaplan-Meier Plot of Overall Survival for Patients with AML
Abbreviations: CI = confidence interval; OS = overall survival; LDAC = low-dose cytarabine.
16. How is Daurismo supplied
DAURISMO is supplied in the following strengths and package configurations:
|
DAURISMO film-coated tablets |
|||
|
Package Configuration |
Tablet Strength (mg) |
NDC |
Print (description) |
|
30 count bottle |
100 mg |
0069-1531-30 |
100 mg strength: 11 mm round, pale orange film-coated tablet debossed with "Pfizer" on one side and "GLS 100" on the other |
|
60 count bottle |
25 mg |
0069-0298-60 |
25 mg strength: 7 mm round, yellow film-coated tablet debossed with "Pfizer" on one side and "GLS 25" on the other |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Embryo-Fetal Toxicity
- •
- Advise female patients of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy. Advise female patients and female partners of male patients to contact their healthcare provider with a known or suspected pregnancy [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
Females and Males of Reproductive Potential
- •
- Advise females of reproductive potential to use effective contraception during treatment with DAURISMO and for at least 30 days after the last dose.
- •
- Advise males of the potential risk of exposure through semen and to use effective contraception, including a condom, even after a vasectomy, to avoid drug exposure to a pregnant partner or a female partner of reproductive potential during treatment with DAURISMO and for at least 30 days after the last dose [see Use in Specific Populations (8.3)].
Semen Donation
- •
- Advise males not to donate semen during treatment with DAURISMO and for at least 30 days after the last dose of DAURISMO[see Use in Specific Populations (8.3)].
Lactation
- •
- Advise women not to breastfeed during treatment with DAURISMO and for at least 30 days after the last dose of DAURISMO [see Use in Specific Populations (8.2)].
Blood Donation
- •
- Advise patients not to donate blood or blood products during treatment with DAURISMO and for at least 30 days after the last dose of DAURISMO [see Warnings and Precautions (5.1)].
Infertility
- •
- Advise males of reproductive potential of the potential for impaired fertility from DAURISMO. Advise male patients to seek advice on effective fertility preservation before treatment [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
QTc Interval Prolongation
- •
- Inform patients of signs and symptoms that may be indicative of significant QTc interval prolongation. Advise patients to contact their healthcare provider immediately in the event of syncope, pre-syncopal symptoms, and cardiac palpitations [see Warnings and Precautions (5.2)].
Musculoskeletal Adverse Reactions
- •
- Advise patients starting therapy with DAURISMO of the risk of muscle-related adverse reactions.
- •
- Advise patients to report promptly any new unexplained muscle pain, tenderness, or weakness occurring during treatment or that persists after discontinuing DAURISMO [see Warnings and Precautions (5.3)].
This product's label may have been updated. For current full prescribing information, please visit www.DAURISMO.com.
LAB-1284-3.0
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised: 3/2023 |
|
MEDICATION GUIDE
|
|
For males:
Exposure to DAURISMO during pregnancy:
|
|
|
What is DAURISMO?
It is not known if DAURISMO is safe and effective in children. |
|
|
Before you take DAURISMO, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements. |
|
|
How should I take DAURISMO?
|
|
|
What should I avoid while taking DAURISMO?
|
|
|
What are the possible side effects of DAURISMO?
The most common side effects of DAURISMO with cytarabine include: |
|
|
|
|
DAURISMO may affect fertility in males. Talk to your healthcare provider if this is a concern for you. |
|
|
How should I store DAURISMO?
Keep DAURISMO and all medicines out of the reach of children. |
|
|
General information about the safe and effective use of DAURISMO.
|
|
|
What are the ingredients in DAURISMO?
 LAB-1285-3.0 |
|
| DAURISMO
glasdegib tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| DAURISMO
glasdegib tablet, film coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Laboratories Div Pfizer Inc | 001147495 | ANALYSIS(0069-0298, 0069-1531) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | PACK(0069-0298, 0069-1531) , LABEL(0069-0298, 0069-1531) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 985052076 | ANALYSIS(0069-0298, 0069-1531) , API MANUFACTURE(0069-0298, 0069-1531) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Deutschland GmbH | 341970073 | ANALYSIS(0069-0298, 0069-1531) , MANUFACTURE(0069-0298, 0069-1531) , PACK(0069-0298, 0069-1531) , LABEL(0069-0298, 0069-1531) | |