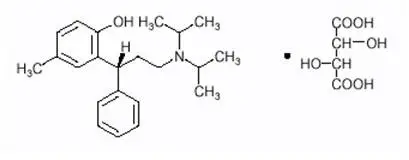
Drug Detail:Detrol (Tolterodine [ tol-ter-oh-deen ])
Drug Class: Urinary antispasmodics
Detrol - Clinical Pharmacology
Tolterodine is a competitive muscarinic receptor antagonist. Both urinary bladder contraction and salivation are mediated via cholinergic muscarinic receptors.
After oral administration, tolterodine is metabolized in the liver, resulting in the formation of the 5-hydroxymethyl derivative, a major pharmacologically active metabolite. The 5-hydroxymethyl metabolite, which exhibits an antimuscarinic activity similar to that of tolterodine, contributes significantly to the therapeutic effect. Both tolterodine and the 5-hydroxymethyl metabolite exhibit a high specificity for muscarinic receptors, since both show negligible activity or affinity for other neurotransmitter receptors and other potential cellular targets, such as calcium channels.
Tolterodine has a pronounced effect on bladder function. Effects on urodynamic parameters before and 1 and 5 hours after a single 6.4 mg dose of tolterodine immediate release were determined in healthy volunteers. The main effects of tolterodine at 1 and 5 hours were an increase in residual urine, reflecting an incomplete emptying of the bladder, and a decrease in detrusor pressure. These findings are consistent with an antimuscarinic action on the lower urinary tract.
Pharmacokinetics
Metabolism
Tolterodine is extensively metabolized by the liver following oral dosing. The primary metabolic route involves the oxidation of the 5-methyl group and is mediated by the cytochrome P450 2D6 (CYP2D6) and leads to the formation of a pharmacologically active 5-hydroxymethyl metabolite. Further metabolism leads to formation of the 5-carboxylic acid and N-dealkylated 5-carboxylic acid metabolites, which account for 51% ± 14% and 29% ± 6.3% of the metabolites recovered in the urine, respectively.
Variability in Metabolism
A subset (about 7%) of the population is devoid of CYP2D6, the enzyme responsible for the formation of the 5-hydroxymethyl metabolite of tolterodine. The identified pathway of metabolism for these individuals ("poor metabolizers") is dealkylation via cytochrome P450 3A4 (CYP3A4) to N-dealkylated tolterodine. The remainder of the population is referred to as "extensive metabolizers." Pharmacokinetic studies revealed that tolterodine is metabolized at a slower rate in poor metabolizers than in extensive metabolizers; this results in significantly higher serum concentrations of tolterodine and in negligible concentrations of the 5-hydroxymethyl metabolite.
Cardiac Electrophysiology
The effect of 2 mg BID and 4 mg BID of tolterodine immediate release (IR) on the QT interval was evaluated in a 4-way crossover, double-blind, placebo- and active-controlled (moxifloxacin 400 mg QD) study in healthy male (N=25) and female (N=23) volunteers aged 18–55 years. Study subjects [approximately equal representation of CYP2D6 extensive metabolizers (EMs) and poor metabolizers (PMs)] completed sequential 4-day periods of dosing with moxifloxacin 400 mg QD, tolterodine 2 mg BID, tolterodine 4 mg BID, and placebo. The 4 mg BID dose of tolterodine IR (two times the highest recommended dose) was chosen because this dose results in tolterodine exposure similar to that observed upon coadministration of tolterodine 2 mg BID with potent CYP3A4 inhibitors in patients who are CYP2D6 poor metabolizers (see PRECAUTIONS, Drug Interactions). QT interval was measured over a 12-hour period following dosing, including the time of peak plasma concentration (Tmax) of tolterodine and at steady state (Day 4 of dosing).
Table 2 summarizes the mean change from baseline to steady state in corrected QT interval (QTc) relative to placebo at the time of peak tolterodine (1 hour) and moxifloxacin (2 hour) concentrations. Both Fridericia's (QTcF) and a population-specific (QTcP) method were used to correct QT interval for heart rate. No single QT correction method is known to be more valid than others. QT interval was measured manually and by machine, and data from both are presented. The mean increase of heart rate associated with a 4 mg/day dose of tolterodine in this study was 2.0 beats/minute and 6.3 beats/minute with 8 mg/day tolterodine. The change in heart rate with moxifloxacin was 0.5 beats/minute.
| Drug/Dose | N | QTcF (msec) (manual) | QTcF (msec) (machine) | QTcP (msec) (manual) | QTcP (msec) (machine) |
|---|---|---|---|---|---|
|
|||||
| Tolterodine 2 mg BID* | 48 | 5.01 (0.28, 9.74) | 1.16 (-2.99, 5.30) | 4.45 (-0.37, 9.26) | 2.00 (-1.81, 5.81) |
| Tolterodine 4 mg BID* | 48 | 11.84 (7.11, 16.58) | 5.63 (1.48, 9.77) | 10.31 (5.49, 15.12) | 8.34 (4.53, 12.15) |
| Moxifloxacin 400 mg QD† | 45 | 19.26‡
(15.49, 23.03) | 8.90 (4.77, 13.03) | 19.10‡
(15.32, 22.89) | 9.29 (5.34, 13.24) |
The reason for the difference between machine and manual read of QT interval is unclear.
The QT effect of tolterodine immediate release tablets appeared greater for 8 mg/day (two times the therapeutic dose) compared to 4 mg/day. The effect of tolterodine 8 mg/day was not as large as that observed after four days of therapeutic dosing with the active control moxifloxacin. However, the confidence intervals overlapped.
Tolterodine's effect on QT interval was found to correlate with plasma concentration of tolterodine. There appeared to be a greater QTc interval increase in CYP2D6 poor metabolizers than in CYP2D6 extensive metabolizers after tolterodine treatment in this study.
This study was not designed to make direct statistical comparisons between drugs or dose levels. There has been no association of Torsade de Pointes in the international post-marketing experience with DETROL or DETROL LA (see PRECAUTIONS, Patients with Congenital or Acquired QT Prolongation).
Related/similar drugs
oxybutynin, Myrbetriq, solifenacin, tolterodine, mirabegron, Botox, DitropanClinical Studies
DETROL Tablets were evaluated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency in four randomized, double-blind, placebo-controlled, 12-week studies. A total of 853 patients received DETROL 2 mg twice daily and 685 patients received placebo. The majority of patients were Caucasian (95%) and female (78%), with a mean age of 60 years (range, 19 to 93 years). At study entry, nearly all patients perceived they had urgency and most patients had increased frequency of micturitions and urge incontinence. These characteristics were well balanced across treatment groups for the studies.
The efficacy endpoints for study 007 (see Table 3) included the change from baseline for:
- Number of incontinence episodes per week
- Number of micturitions per 24 hours (averaged over 7 days)
- Volume of urine voided per micturition (averaged over 2 days)
The efficacy endpoints for studies 008, 009, and 010 (see Table 4) were identical to the above endpoints with the exception that the number of incontinence episodes was per 24 hours (averaged over 7 days).
| DETROL (SD) N=514 | Placebo (SD) N=508 | Difference (95% CI) |
|
|---|---|---|---|
| SD = Standard Deviation. | |||
|
|||
| Number of Incontinence Episodes per Week | |||
| Mean baseline | 23.2 | 23.3 | |
| Mean change from baseline | -10.6 (17) | -6.9 (15) | -3.7 (-5.7, -1.6) |
| Number of Micturitions per 24 Hours | |||
| Mean baseline | 11.1 | 11.3 | |
| Mean change from baseline | -1.7 (3.3) | -1.2 (2.9) | -0.5* (-0.9, -0.1) |
| Volume Voided per Micturition (mL) | |||
| Mean baseline | 137 | 136 | |
| Mean change from baseline | 29 (47) | 14 (41) | 15* (9, 21) |
| Study | DETROL (SD) | Placebo (SD) | Difference (95% CI) |
|
|---|---|---|---|---|
| SD = Standard Deviation. | ||||
|
||||
| Number of Incontinence Episodes per 24 Hours | ||||
| 008 | Number of patients | 93 | 40 | |
| Mean baseline | 2.9 | 3.3 | ||
| Mean change from baseline | -1.3 (3.2) | -0.9 (1.5) | 0.5 (-1.3,0.3) | |
| 009 | Number of patients | 116 | 55 | |
| Mean baseline | 3.6 | 3.5 | ||
| Mean change from baseline | -1.7 (2.5) | -1.3 (2.5) | -0.4 (-1.0,0.2) | |
| 010 | Number of patients | 90 | 50 | |
| Mean baseline | 3.7 | 3.5 | ||
| Mean change from baseline | -1.6 (2.4) | -1.1 (2.1) | -0.5 (-1.1,0.1) | |
| Number of Micturitions per 24 Hours | ||||
| 008 | Number of patients | 118 | 56 | |
| Mean baseline | 11.5 | 11.7 | ||
| Mean change from baseline | -2.7 (3.8) | -1.6 (3.6) | -1.2* (-2.0,-0.4) | |
| 009 | Number of patients | 128 | 64 | |
| Mean baseline | 11.2 | 11.3 | ||
| Mean change from baseline | -2.3 (2.1) | -1.4 (2.8) | -0.9* (-1.5,-0.3) | |
| 010 | Number of patients | 108 | 56 | |
| Mean baseline | 11.6 | 11.6 | ||
| Mean change from baseline | -1.7 (2.3) | -1.4 (2.8) | -0.38 (-1.1,0.3) | |
| Volume Voided per Micturition (mL) | ||||
| 008 | Number of patients | 118 | 56 | |
| Mean baseline | 166 | 157 | ||
| Mean change from baseline | 38 (54) | 6 (42) | 32* (18,46) | |
| 009 | Number of patients | 129 | 64 | |
| Mean baseline | 155 | 158 | ||
| Mean change from baseline | 36 (50) | 10 (47) | 26* (14,38) | |
| 010 | Number of patients | 108 | 56 | |
| Mean baseline | 155 | 160 | ||
| Mean change from baseline | 31 (45) | 13 (52) | 18* (4,32) | |
Contraindications
DETROL Tablets are contraindicated in patients with urinary retention, gastric retention, or uncontrolled narrow-angle glaucoma. DETROL is also contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients, or to fesoterodine fumarate extended-release tablets which, like DETROL, are metabolized to 5-hydroxymethyl tolterodine.
Precautions
General
Patients with Congenital or Acquired QT Prolongation
In a study of the effect of tolterodine immediate release tablets on the QT interval (see CLINICAL PHARMACOLOGY, Cardiac Electrophysiology), the effect on the QT interval appeared greater for 8 mg/day (two times the therapeutic dose) compared to 4 mg/day and was more pronounced in CYP2D6 poor metabolizers (PM) than extensive metabolizers (EMs). The effect of tolterodine 8 mg/day was not as large as that observed after four days of therapeutic dosing with the active control moxifloxacin. However, the confidence intervals overlapped. These observations should be considered in clinical decisions to prescribe DETROL for patients with a known history of QT prolongation or patients who are taking Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications (see PRECAUTIONS, Drug Interactions). There has been no association of Torsade de Pointes in the international post-marketing experience with DETROL or DETROL LA.
Geriatric Use
Of the 1120 patients who were treated in the four Phase 3, 12-week clinical studies of DETROL, 474 (42%) were 65 to 91 years of age. No overall differences in safety were observed between the older and younger patients (see CLINICAL PHARMACOLOGY, Pharmacokinetics in Special Populations).
Adverse Reactions/Side Effects
The Phase 2 and 3 clinical trial program for DETROL Tablets included 3071 patients who were treated with DETROL (N=2133) or placebo (N=938). The patients were treated with 1, 2, 4, or 8 mg/day for up to 12 months. No differences in the safety profile of tolterodine were identified based on age, gender, race, or metabolism.
The data described below reflect exposure to DETROL 2 mg bid in 986 patients and to placebo in 683 patients exposed for 12 weeks in five Phase 3, controlled clinical studies. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and approximating rates.
Sixty-six percent of patients receiving DETROL 2 mg bid reported adverse events versus 56% of placebo patients. The most common adverse events reported by patients receiving DETROL were dry mouth, headache, constipation, vertigo/dizziness, and abdominal pain. Dry mouth, constipation, abnormal vision (accommodation abnormalities), urinary retention, and xerophthalmia are expected side effects of antimuscarinic agents.
Dry mouth was the most frequently reported adverse event for patients treated with DETROL 2 mg bid in the Phase 3 clinical studies, occurring in 34.8% of patients treated with DETROL and 9.8% of placebo-treated patients. One percent of patients treated with DETROL discontinued treatment due to dry mouth.
The frequency of discontinuation due to adverse events was highest during the first 4 weeks of treatment. Seven percent of patients treated with DETROL 2 mg bid discontinued treatment due to adverse events versus 6% of placebo patients. The most common adverse events leading to discontinuation of DETROL were dizziness and headache.
Three percent of patients treated with DETROL 2 mg bid reported a serious adverse event versus 4% of placebo patients. Significant ECG changes in QT and QTc have not been demonstrated in clinical-study patients treated with DETROL 2 mg bid. Table 5 lists the adverse events reported in 1% or more of the patients treated with DETROL 2 mg bid in the 12-week studies. The adverse events are reported regardless of causality.
| Body System | Adverse Event | % DETROL N=986 | % Placebo N=683 |
|---|---|---|---|
|
|||
| Autonomic Nervous | accommodation abnormal | 2 | 1 |
| dry mouth | 35 | 10 | |
| General | chest pain | 2 | 1 |
| fatigue | 4 | 3 | |
| headache | 7 | 5 | |
| influenza-like symptoms | 3 | 2 | |
| Central/Peripheral Nervous | vertigo/dizziness | 5 | 3 |
| Gastrointestinal | abdominal pain | 5 | 3 |
| constipation | 7 | 4 | |
| diarrhea | 4 | 3 | |
| dyspepsia | 4 | 1 | |
| Urinary | dysuria | 2 | 1 |
| Skin/Appendages | dry skin | 1 | 0 |
| Musculoskeletal | arthralgia | 2 | 1 |
| Vision | xerophthalmia | 3 | 2 |
| Psychiatric | somnolence | 3 | 2 |
| Metabolic/Nutritional | weight gain | 1 | 0 |
| Resistance Mechanism | infection | 1 | 0 |
Detrol Dosage and Administration
The initial recommended dose of DETROL Tablets is 2 mg twice daily. The dose may be lowered to 1 mg twice daily based on individual response and tolerability. For patients with significantly reduced hepatic or renal function or who are currently taking drugs that are potent inhibitors of CYP3A4, the recommended dose of DETROL is 1 mg twice daily (see PRECAUTIONS, General, PRECAUTIONS, Reduced Hepatic and Renal Function, and PRECAUTIONS, Drug Interactions).
| DETROL
tolterodine tartrate tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| DETROL
tolterodine tartrate tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Pharmaceuticals LLC | 829084545 | ANALYSIS(0009-4541, 0009-4544) , API MANUFACTURE(0009-4541, 0009-4544) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NEOLPHARMA, INC. | 078709787 | ANALYSIS(0009-4541, 0009-4544) , LABEL(0009-4541, 0009-4544) , MANUFACTURE(0009-4541, 0009-4544) , PACK(0009-4541, 0009-4544) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-4541, 0009-4544) , API MANUFACTURE(0009-4541, 0009-4544) , PACK(0009-4541, 0009-4544) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Italia S.r.l. | 458521908 | ANALYSIS(0009-4541, 0009-4544) , MANUFACTURE(0009-4541, 0009-4544) , PACK(0009-4541, 0009-4544) | |