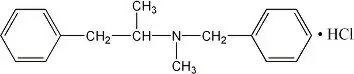
Drug Detail:Didrex (Benzphetamine [ benz-fet-ah-meen ])
Drug Class: Anorexiants CNS stimulants
Didrex - Clinical Pharmacology
Benzphetamine hydrochloride is a sympathomimetic amine with pharmacologic activity similar to the prototype drugs of this class used in obesity, the amphetamines. Actions include central nervous system stimulation and elevation of blood pressure. Tachyphylaxis and tolerance have been demonstrated with all drugs of this class in which these phenomena have been looked for.
Drugs of this class used in obesity are commonly known as "anorectics" or "anorexigenics". It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions, or metabolic effects, may be involved.
Adult obese subjects instructed in dietary management and treated with "anorectic" drugs, lose more weight on the average than those treated with placebo and diet, as determined in relatively short-term clinical trials.
The magnitude of increased weight loss of drug-treated patients over placebo-treated patients is only a fraction of a pound a week. The rate of weight loss is the greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The possible origins of the increased weight loss due to the various drug effects are not established. The amount of weight loss associated with the use of an "anorectic" drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician-investigator, the population treated, and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured in years, whereas the studies cited are restricted to a few weeks duration; thus, the total impact of drug-induced weight loss over that of diet alone must be considered to be clinically limited.
Pharmacokinetic data in humans are not available.
Contraindications
DIDREX Tablets are contraindicated in patients with advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension, hyperthyroidism, known hypersensitivity or idiosyncrasy to sympathomimetic amines, and glaucoma. Benzphetamine should not be given to patients who are in an agitated state or who have a history of drug abuse.
Hypertensive crises have resulted when sympathomimetic amines have been used concomitantly or within 14 days following use of monoamine oxidase inhibitors. DIDREX should not be used concomitantly with other CNS stimulants.
DIDREX may cause fetal harm when administered to a pregnant woman. Amphetamines have been shown to be teratogenic and embryotoxic in mammals at high multiples of the human dose. DIDREX is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Warnings
DIDREX Tablets should not be used in combination with other anorectic agents, including prescribed drugs, over-the-counter preparations and herbal products.
In a case-control epidemiological study, the use of anorectic agents was associated with an increased risk of developing pulmonary hypertension, a rare, but often fatal disorder. The use of anorectic agents for longer than three months was associated with a 23-fold increase in the risk of developing pulmonary hypertension. Increased risk of pulmonary hypertension with repeated courses of therapy cannot be excluded. It should be noted that benzphetamine was not specifically studied in this case-control study.
The onset or aggravation of exertional dyspnea, or unexplained symptoms of angina pectoris, syncope, or lower extremity edema suggest the possibility of occurrence of pulmonary hypertension. Under these circumstances, DIDREX Tablets should be immediately discontinued, and the patient should be evaluated for the possible presence of pulmonary hypertension.
Valvular heart disease associated with the use of some anorectic agents such as fenfluramine and dexfenfluramine has been reported. Possible contributing factors include use for extended periods of time, higher than recommended dose, and/or use in combination with other anorectic drugs. However, no cases of this valvulopathy have been reported when benzphetamine has been used alone.
The potential risk of possible serious adverse effects such as valvular heart disease and pulmonary hypertension should be assessed carefully against the potential benefit of weight loss. Baseline cardiac evaluation should be considered to detect pre-existing valvular heart diseases or pulmonary hypertension prior to initiation of benzphetamine treatment. DIDREX Tablets are not recommended in patients with known heart murmur or valvular heart disease. Echocardiogram during and after treatment could be useful for detecting any valvular disorders which may occur. To limit unwarranted exposure and risks, treatment with DIDREX Tablets should be continued only if the patient has satisfactory weight loss within the first 4 weeks of treatment (i.e., weight loss of at least 4 pounds, or as determined by the physician and patient).
When tolerance to the anorectic effect develops, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinued.
DIDREX Tablets are not recommended for severely hypertensive patients or for patients with symptomatic cardiovascular disease including arrhythmias.
DIDREX Tablets are not recommended for patients who used any anorectic agents within the prior year.
| DIDREX
benzphetamine hydrochloride tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmacia and Upjohn Company (829076566) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Puerto Rico Inc | 143814544 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia and Upjohn Company | 829076566 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Warner Lambert Company LLC | 001344506 | ANALYSIS | |