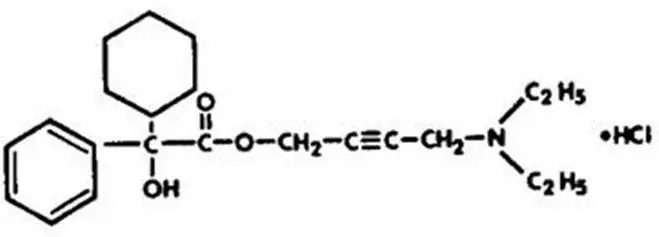Drug Detail:Ditropan xl (Oxybutynin (oral) [ ox-i-bue-ti-nin ])
Drug Class: Urinary antispasmodics
Ditropan - Clinical Pharmacology
Oxybutynin chloride exerts a direct antispasmodic effect on smooth muscle and inhibits the muscarinic action of acetylcholine on smooth muscle. Oxybutynin chloride exhibits only one fifth of the anticholinergic activity of atropine on the rabbit detrusor muscle, but four to ten times the antispasmodic activity. No blocking effects occur at skeletal neuromuscular junctions or autonomic ganglia (antinicotinic effects).
Oxybutynin chloride relaxes bladder smooth muscle. In patients with conditions characterized by involuntary bladder contractions, cystometric studies have demonstrated that oxybutynin chloride increases bladder (vesical) capacity, diminishes the frequency of uninhibited contractions of the detrusor muscle, and delays the initial desire to void. Oxybutynin chloride thus decreases urgency and the frequency of both incontinent episodes and voluntary urination.
Antimuscarinic activity resides predominately in the R-isomer. A metabolite, desethyloxybutynin, has pharmacological activity similar to that of oxybutynin in in vitro studies.
Pharmacokinetics
Absorption
Following oral administration of DITROPAN, oxybutynin is rapidly absorbed achieving Cmax within an hour, following which plasma concentration decreases with an effective half-life of approximately 2 to 3 hours. The absolute bioavailability of oxybutynin is reported to be about 6% (range 1.6 to 10.9%) for the tablets. Wide interindividual variation in pharmacokinetic parameters is evident following oral administration of oxybutynin.
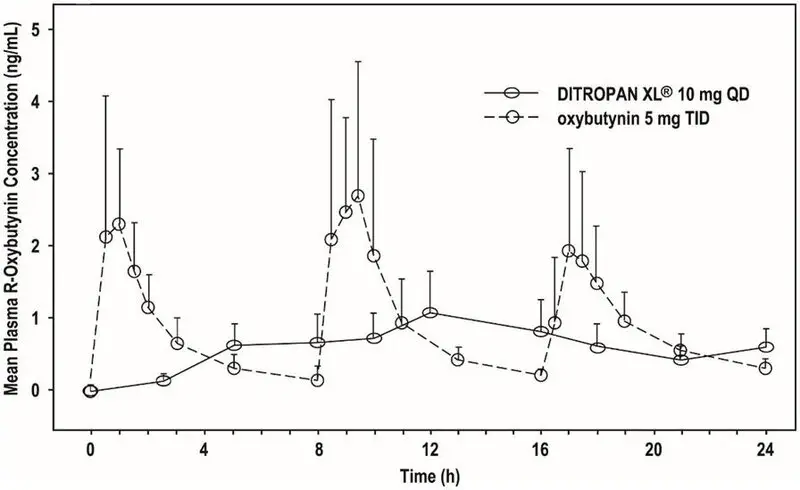
The mean pharmacokinetic parameters for R- and S-oxybutynin are summarized in Table 1. The plasma concentration-time profiles for R- and S-oxybutynin are similar in shape; Figure 1 shows the profile for R-oxybutynin.
| Parameters (units) | R-Oxybutynin | S-Oxybutynin |
|---|---|---|
| Cmax (ng/mL) | 3.6 (2.2) | 7.8 (4.1) |
| Tmax (h) | 0.89 (0.34) | 0.65 (0.32) |
| AUCt (ng∙h/mL) | 22.6 (11.3) | 35.0 (17.3) |
| AUCinf (ng∙h/mL) | 24.3 (12.3) | 37.3 (18.7) |
| Figure 1. Mean R-oxybutynin plasma concentrations following three doses of DITROPAN 5 mg administered every 8 hours for 1 day in 23 healthy adult volunteers |
 |
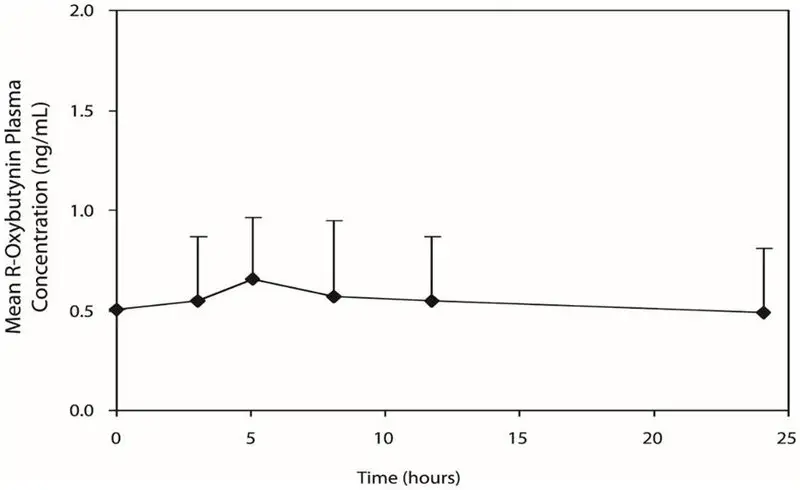
DITROPAN steady-state pharmacokinetics were also studied in 11 pediatric patients with detrusor overactivity associated with a neurological condition (e.g., spina bifida). These pediatric patients were on DITROPAN tablets with total daily dose ranging from 7.5 mg to 15 mg (0.22 to 0.53 mg/kg). Overall, most patients (86.9%) were taking a total daily DITROPAN dose between 10 mg and 15 mg. Sparse sampling technique was used to obtain serum samples. When all available data are normalized to an equivalent of 5 mg twice daily DITROPAN, the mean pharmacokinetic parameters derived for R- and S-oxybutynin and R- and S-desethyloxybutynin are summarized in Table 2. The plasma-time concentration profiles for R- and S-oxybutynin are similar in shape; Figure 2 shows the profile for R-oxybutynin when all available data are normalized to an equivalent of 5 mg twice daily.
| All Available Data Normalized to an Equivalent of DITROPAN Tablets 5 mg BID or TID at Steady State | ||||
|---|---|---|---|---|
| R-Oxybutynin | S-Oxybutynin | R- Desethyloxybutynin | S- Desethyloxybutynin | |
|
||||
| Cmax* (ng/mL) | 6.1± 3.2 | 10.1 ± 7.5 | 55.4 ± 17.9 | 28.2 ± 10.0 |
| Tmax (hr) | 1.0 | 1.0 | 2.0 | 2.0 |
| AUC†
(ng.hr/mL) | 19.8 ± 7.4 | 28.4 ± 12.7 | 238.8 ± 77.6 | 119.5 ± 50.7 |
| Figure 2. Mean steady-state (±SD) R-oxybutynin plasma concentrations following administration of total daily DITROPAN Tablet dose of 7.5 mg to 15 mg (0.22 mg/kg to 0.53 mg/kg) in children 5–15 years of age. – Plot represents all available data normalized to the equivalent of DITROPAN 5 mg BID or TID at steady state |
 |
Contraindications
DITROPAN® (oxybutynin chloride) is contraindicated in patients with urinary retention, gastric retention and other severe decreased gastrointestinal motility conditions, uncontrolled narrow-angle glaucoma and in patients who are at risk for these conditions.
DITROPAN is also contraindicated in patients who have demonstrated hypersensitivity to the drug substance or other components of the product.
Adverse Reactions/Side Effects
The safety and efficacy of DITROPAN® (oxybutynin chloride) was evaluated in a total of 199 patients in three clinical trials. These participants were treated with DITROPAN 5–20 mg/day for up to 6 weeks. Table 3 shows the incidence of adverse events judged by investigators to be at least possibly related to treatment and reported by at least 5% of patients.
| Body System | Adverse Event | DITROPAN (5–20 mg/day) (n=199) |
|---|---|---|
| Infections and Infestations | Urinary tract infection | 6.5% |
| Psychiatric Disorders | Insomnia | 5.5% |
| Nervousness | 6.5% | |
| Nervous System Disorders | Dizziness | 16.6% |
| Somnolence | 14.0% | |
| Headache | 7.5% | |
| Eye Disorders | Blurred vision | 9.6% |
| Gastrointestinal Disorders | Dry mouth | 71.4% |
| Constipation | 15.1% | |
| Nausea | 11.6% | |
| Dyspepsia | 6.0% | |
| Renal and Urinary Disorders | Urinary Hesitation | 8.5% |
| Urinary Retention | 6.0% |
The most common adverse events reported by patients receiving DITROPAN 5–20 mg/day were the expected side effects of anticholinergic agents. The incidence of dry mouth was dose-related.
In addition, the following adverse events were reported by 1 to <5% of patients using DITROPAN (5–20 mg/day) in all studies. Infections and Infestations: nasopharyngitis, upper respiratory tract infection, bronchitis, cystitis, fungal infection; Metabolism and Nutrition Disorders: fluid retention; Psychiatric Disorders: confusional state; Nervous System Disorders: dysgeusia, sinus headache; Eye Disorders: keratoconjunctivitis sicca, eye irritation; Cardiac Disorders: palpitations, sinus arrhythmia; Vascular Disorders: flushing; Respiratory, Thoracic and Mediastinal Disorders: nasal dryness, cough, pharyngolaryngeal pain, dry throat, sinus congestion, hoarseness, asthma, nasal congestion; Gastrointestinal Disorders: diarrhea, abdominal pain, loose stools, flatulence, vomiting, abdominal pain upper, dysphagia, aptyalism, eructation, tongue coated; Skin and Subcutaneous Tissue Disorders: dry skin, pruritis; Musculoskeletal and Connective Tissue Disorders: back pain, arthralgia, pain in extremity, flank pain; Renal and Urinary Disorders: dysuria, pollakiuria; General Disorders and Administration Site Conditions: fatigue, edema peripheral, asthenia, pain, thirst, edema; Investigations: blood pressure increased, blood glucose increased, blood pressure decreased; Injury, Poisoning, and Procedural Complications: fall.
| DITROPAN
oxybutynin chloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - ALZA Corporation (175417641) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| sanofi aventis | 783243835 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PCAS France (API) | 396133998 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Ortho LLC | 084894661 | ANALYSIS | |