Drug Detail:Duexis (Famotidine and ibuprofen [ fam-oh-ti-deen-and-eye-bue-proe-fen ])
Drug Class: Nonsteroidal anti-inflammatory drugs
Highlights of Prescribing Information
DUEXIS (ibuprofen and famotidine) tablets, for oral use
Initial U.S. Approval: 2011
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
See full prescribing information for complete boxed warning.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use (5.1)
- DUEXIS is contraindicated in the setting of coronary artery bypass graft (CABG) surgery (4, 5.1)
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events (5.2)
Recent Major Changes
| 4/2021 |
| 4/2021 |
Indications and Usage for Duexis
DUEXIS, a combination of a nonsteroidal anti-inflammatory drug (NSAID) ibuprofen and the histamine H2-receptor antagonist famotidine, is indicated for the relief of signs and symptoms of rheumatoid arthritis and osteoarthritis and to decrease the risk of developing upper gastrointestinal ulcers, which in the clinical trials was defined as a gastric and/or duodenal ulcer, in patients who are taking ibuprofen for those indications. The clinical trials primarily enrolled patients less than 65 years of age without a prior history of gastrointestinal ulcer. Controlled trials do not extend beyond 6 months. (1)
Duexis Dosage and Administration
- One DUEXIS tablet administered orally three times per day. (2)
- Use ibuprofen at the lowest effective dosage for the shortest duration consistent with individual patient treatment goals. (2)
- Do not substitute DUEXIS with the single-ingredient products of ibuprofen and famotidine. (2)
Dosage Forms and Strengths
- DUEXIS (ibuprofen and famotidine) Tablets: 800 mg ibuprofen and 26.6 mg famotidine. (3)
Contraindications
- Known hypersensitivity to ibuprofen or famotidine or any components of the drug product. (4)
- History of asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. (4)
- In the setting of CABG surgery. (4)
- Known hypersensitivity to other H2-receptor antagonists. (4)
Warnings and Precautions
- Hepatotoxicity: Inform patients of warning signs and symptoms of hepatotoxicity. Discontinue if abnormal liver tests persist or worsen or if clinical signs and symptoms of liver disease develop. (5.4)
- Hypertension: Patients taking some antihypertensive medications may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure. (5.5, 7)
- Heart Failure and Edema: Avoid use of DUEXIS in patients with severe heart failure unless benefits are expected to outweigh risk of worsening heart failure. (5.6)
- Active Bleeding: Active and clinically significant bleeding from any source can occur; discontinue DUEXIS if active bleeding occurs. (5.3)
- Renal Toxicity: Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of DUEXIS in patients with advanced renal disease unless benefits are expected to outweigh risk of worsening renal function. (5.7)
- Anaphylactic Reactions: Seek emergency help if an anaphylactic reaction occurs. (5.8)
- Exacerbation of Asthma Related to Aspirin Sensitivity: DUEXIS is contraindicated in patients with aspirin-sensitive asthma. Monitor patients with preexisting asthma (without aspirin-sensitivity). (5.10)
- Serious Skin Reactions: Discontinue DUEXIS at first appearance of skin rash or other signs of hypersensitivity (5.11).
- Drug Reaction with Eosinophilia and Systematic Symptoms (DRESS): Discontinue and evaluate clinically (5.12).
- Fetal Toxicity: Limit use of NSAIDs, including DUEXIS, between about 20 to 30 weeks in pregnancy due to the risk of oligohydramnios/fetal renal dysfunction. Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy due to the risks of oligohydramnios/fetal renal dysfunction and premature closure of the fetal ductus arteriosus (5.13, 8.1)
- Hematologic Toxicity: Monitor hemoglobin or hematocrit in patient with any signs or symptoms of anemia. (5.14)
Adverse Reactions/Side Effects
Most common adverse reactions (≥1% and greater than ibuprofen alone) are nausea, diarrhea, constipation, upper abdominal pain, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Horizon at (1-866-479-6742) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
See full prescribing information for a list of clinically important drug interactions. (7)
Use In Specific Populations
- Pregnancy: Use of NSAIDs during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs in pregnant women starting at 30 weeks gestation. (5.13, 8.1)
- Females and Males of Reproductive Potential: NSAIDs are associated with reversible infertility. Consider withdrawal of DUEXIS in women who have difficulties conceiving. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2021
Full Prescribing Information
1. Indications and Usage for Duexis
DUEXIS, a combination of the NSAID ibuprofen and the histamine H2-receptor antagonist famotidine, is indicated for the relief of signs and symptoms of rheumatoid arthritis and osteoarthritis and to decrease the risk of developing upper gastrointestinal ulcers, which in the clinical trials was defined as a gastric and/or duodenal ulcer, in patients who are taking ibuprofen for those indications. The clinical trials primarily enrolled patients less than 65 years of age without a prior history of gastrointestinal ulcer. Controlled trials do not extend beyond 6 months [see Clinical Studies (14), Use in Specific Populations (8.5)].
2. Duexis Dosage and Administration
Carefully consider the potential benefits and risks of DUEXIS and other treatment options before deciding to use DUEXIS. Use ibuprofen at the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)].
The recommended daily dose of DUEXIS (ibuprofen and famotidine) 800 mg/26.6 mg is a single tablet administered orally three times per day.
DUEXIS tablets should be swallowed whole, and should not be cut to supply a lower dose. Do not chew, divide, or crush tablets.
Patients should be instructed that if a dose is missed, it should be taken as soon possible. However, if the next scheduled dose is due, the patient should not take the missed dose, and should be instructed to take the next dose on time. Patients should be instructed not to take 2 doses at one time to make up for a missed dose.
Do not substitute DUEXIS with the single-ingredient products of ibuprofen and famotidine.
3. Dosage Forms and Strengths
DUEXIS (ibuprofen and famotidine) tablets: 800 mg/26.6 mg, are light blue, oval, biconvex, film-coated tablets debossed with "HZT" on one side.
4. Contraindications
DUEXIS is contraindicated in the following patients:
- Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to ibuprofen or famotidine or any components of the drug product [see Warnings and Precautions (5.8, 5.11)].
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients [see Warnings and Precautions (5.8, 5.10)].
- In the setting of coronary artery bypass graft (CABG) surgery [see Warnings and Precautions (5.1)].
- DUEXIS should not be administered to patients with a history of hypersensitivity to other H2-receptor antagonists. Cross sensitivity with other H2-receptor antagonists has been observed.
5. Warnings and Precautions
5.1 Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI), and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDS. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as ibuprofen, increases the risk of serious gastrointestinal GI events [see Warnings and Precautions (5.2)].
5.2 Gastrointestinal Bleeding, Ulceration, and Perforation
NSAIDs, including ibuprofen, cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the esophagus, stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDS. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occurred in approximately 1% of patients treated for 3-6 months, and in about 2%-4% of patients treated for one year. However, even short-term NSAID therapy is not without risk.
5.3 Active Bleeding
When active and clinically significant bleeding from any source occurs in patients receiving DUEXIS, the treatment should be withdrawn. Patients with initial hemoglobin values of 10 g or less who are to receive long-term therapy should have hemoglobin values determined periodically.
5.4 Hepatotoxicity
Elevations of ALT or AST (three or more times the upper limit of normal [ULN]) have been reported in approximately 1% of NSAID-treated patients in clinical trials. In addition, rare, sometimes fatal, cases of severe hepatic injury, including fulminant hepatitis, liver necrosis, and hepatic failure have been reported.
Elevations of ALT or AST (less than three times ULN) may occur in up to 15% of patients treated with NSAIDs including ibuprofen.
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), discontinue DUEXIS immediately, and perform a clinical evaluation of the patient.
5.5 Hypertension
NSAIDs, including DUEXIS, can lead to new onset of hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Patients taking angiotensin converting enzyme (ACE) inhibitors, thiazide diuretics, or loop diuretics may have impaired response to these therapies when taking NSAIDs [see Drug Interactions (7)].
Monitor blood pressure (BP) during the initiation of NSAID treatment and throughout the course of therapy.
5.6 Heart Failure and Edema
The Coxib and traditional NSAID Trialists' Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalizations for heart failure in COX-2 selective treated patients and nonselective NSAID-treated patients compared to placebo-treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of ibuprofen may blunt the CV effects of several therapeutic agents used to treat these medical conditions (e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers [ARBs]) [see Drug Interactions (7)].
Avoid the use of DUEXIS in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If DUEXIS is used in patients with severe heart failure, monitor patients for signs and symptoms of worsening heart failure.
5.8 Anaphylactic Reactions
Ibuprofen has been associated with anaphylactic reactions in patients with and without known hypersensitivity to ibuprofen and in patients with aspirin-sensitive asthma [see Contraindications (4), Warnings and Precautions (5.8)].
Seek emergency help if an anaphylactic reaction occurs.
5.9 Seizures
Central nervous system (CNS) adverse effects including seizures, delirium, and coma have been reported with famotidine in patients with moderate (creatinine clearance <50 mL/min) and severe renal insufficiency (creatinine clearance <10 mL/min), and the dosage of the famotidine component in DUEXIS is fixed. Therefore, DUEXIS is not recommended in patients with creatinine clearance < 50 mL/min.
5.10 Exacerbation of Asthma Related to Aspirin Sensitivity
A subpopulation of patients with asthma may have aspirin-sensitive asthma which may include chronic rhinosinusitis complicated by nasal polyps; severe, potentially fatal bronchospasm; and/or intolerance to aspirin and other NSAIDs. Because cross-reactivity between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, DUEXIS is contraindicated in patients with this form of aspirin sensitivity [see Contraindications (4)]. When DUEXIS is used in patients with preexisting asthma (without known aspirin sensitivity), monitor patients for changes in the signs and symptoms of asthma.
5.11 Serious Skin Reactions
NSAIDs, including ibuprofen, which is a component of DUEXIS tablets, can cause serious skin adverse reactions such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions and to discontinue the use of DUEXIS at the first appearance of skin rash or any other sign of hypersensitivity. DUEXIS is contraindicated in patients with previous serious skin reactions to NSAIDs [see Contraindications (4)].
5.12 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as DUEXIS. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, discontinue DUEXIS and evaluate the patient immediately.
5.14 Hematologic Toxicity
Anemia has occurred in NSAID-treated patients. This may be due to occult or gross blood loss, fluid retention, or an incompletely described effect on erythropoiesis. If a patient treated with DUEXIS has any signs or symptoms of anemia, monitor hemoglobin or hematocrit.
NSAIDs, including DUEXIS, may increase the risk of bleeding events. Co-morbid conditions such as coagulation disorders or concomitant use of warfarin, and other anticoagulants, antiplatelet agents (e.g., aspirin), serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may increase the risk. Monitor these patients for signs of bleeding [see Drug Interactions (7)].
5.15 Masking of Inflammation and Fever
The pharmacological activity of DUEXIS in reducing inflammation, and possibly fever, may diminish the utility of diagnostic signs in detecting infections.
5.16 Laboratory Monitoring
Because serious GI bleeding, hepatotoxicity, and renal injury can occur without warning symptoms or signs, consider monitoring patients on long-term NSAID treatment with a CBC and chemistry profile periodically [see Warnings and Precautions (5.2, 5.4, 5.7)].
5.17 Concomitant NSAID Use
DUEXIS contains ibuprofen as one of its active ingredients. It should not be used with other ibuprofen-containing products.
The concomitant use of NSAIDs, including aspirin, with DUEXIS may increase the risk of adverse reactions [see Adverse Reactions (6), Drug Interactions (7), Clinical Studies (14)].
5.18 Aseptic Meningitis
Aseptic meningitis with fever and coma has been observed on rare occasions in patients on ibuprofen, which is a component of DUEXIS. Although it is probably more likely to occur in patients with systemic lupus erythematosus (SLE) and related connective tissue diseases, it has been reported in patients who do not have an underlying chronic disease. If signs or symptoms of meningitis develop in a patient on DUEXIS, the possibility of its being related to ibuprofen should be considered.
5.19 Ophthalmological Effects
Blurred and/or diminished vision, scotomata, and/or changes in color vision have been reported. If a patient develops such complaints while receiving DUEXIS, the drug should be discontinued, and the patient should have an ophthalmologic examination which includes central visual fields and color vision testing.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events [see Warnings and Precautions (5.1)]
- GI Bleeding, Ulceration, and Perforation [see Warnings and Precautions (5.2)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
- Hypertension [see Warnings and Precautions (5.5)]
- Heart Failure and Edema [see Warnings and Precautions (5.6)]
- Renal Toxicity and Hyperkalemia [see Warnings and Precautions (5.7)]
- Anaphylactic Reactions [see Warnings and Precautions (5. 8)]
- Seizures [see Warnings and Precautions (5.9)]
- Serious Skin Reactions [see Warnings and Precautions (5.11)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [see Warnings and Precautions (5.12)]
- Fetal Toxicity [see Warnings and Precautions (5.13)]
- Hematologic Toxicity [see Warnings and Precautions (5.14)]
- Aseptic Meningitis [see Warnings and Precautions (5.18)]
- Ophthalmological Effects [see Warnings and Precautions (5.19)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of DUEXIS was evaluated in 1022 patients in controlled clinical studies, including 508 patients treated for at least 6 months and 107 patients treated for approximately 1 year. Patients treated with DUEXIS ranged in age from 39 to 80 years (median age 55 years), with 67% female, 79% Caucasian, 18% African-American, and 3% other races. Two randomized, active-controlled clinical studies (Study 301 and Study 303) were conducted for the reduction of the risk of development of ibuprofen-associated, upper gastrointestinal ulcers in patients who required use of ibuprofen, which included 1022 patients on DUEXIS and 511 patients on ibuprofen alone. Approximately 15% of patients were on low- dose aspirin. Patients were assigned randomly, in a 2:1 ratio, to treatment with either DUEXIS or ibuprofen 800 mg three times a day for 24 consecutive weeks.
Three serious cases of acute renal failure were observed in patients treated with DUEXIS in the two controlled clinical trials. All three patients recovered to baseline levels after discontinuation of DUEXIS. Additionally, increases in serum creatinine were observed in both treatment arms in the two clinical studies. Many of these patients were taking concomitant diuretics and/or angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers. There were patients with a normal baseline serum creatinine level who developed abnormal values in the controlled trials as presented in Table 1.
| Study 301 | Study 303 | ||||
|---|---|---|---|---|---|
| Baseline | Post-Baseline‡ | DUEXIS N=414 % (n) | Ibuprofen N=207 % (n) | DUEXIS N=598 % (n) | Ibuprofen N=296 % (n) |
|
|||||
| Normal* | Abnormal† | 4% (17) | 2% (4) | 2%(15) | 4% (12) |
7. Drug Interactions
See Table 3 for clinically significant drug interactions with ibuprofen.
| Drugs That Interfere with Hemostasis | |
| Clinical Impact: |
|
| Intervention: | Monitor patients with concomitant use of DUEXIS with anticoagulants (e.g., warfarin), antiplatelet agents (e.g., aspirin), selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) for signs of bleeding [see Warnings and Precautions (5.16)]. |
| Aspirin | |
| Clinical Impact: | Pharmacodynamic (PD) studies have demonstrated interference with the antiplatelet activity of aspirin when ibuprofen 400 mg, given three times daily, is administered with enteric-coated low-dose aspirin. The interaction exists even following a once-daily regimen of ibuprofen 400 mg, particularly when ibuprofen is dosed prior to aspirin. The interaction is alleviated if immediate-release low-dose aspirin is dosed at least 2 hours prior to a once-daily regimen of ibuprofen; however, this finding cannot be extended to enteric-coated low-dose aspirin [see Clinical Pharmacology (12.2)]. |
| Controlled clinical studies showed that the concomitant use of NSAIDs and analgesic doses of aspirin does not produce any greater therapeutic effect than the use of NSAIDs alone. In a clinical study, the concomitant use of an NSAID and aspirin was associated with a significantly increased incidence of GI adverse reactions as compared to use of the NSAID alone [see Warnings and Precautions (5.2)]. | |
| Intervention: | Because there may be an increased risk of cardiovascular events due to the interference of ibuprofen with the antiplatelet effect of aspirin, for patients taking low-dose aspirin for cardioprotection who require analgesics, consider use of an NSAID that does not interfere with the antiplatelet effect of aspirin, or non-NSAID analgesics, where appropriate. |
| Concomitant use of DUEXIS and analgesic doses of aspirin is not generally recommended because of the increased risk of bleeding [see Warnings and Precautions (5.3)]. DUEXIS is not a substitute for low dose aspirin for cardiovascular protection. |
|
| ACE Inhibitors, Angiotensin Receptor Blockers, and Beta-blockers | |
| Clinical Impact: |
|
| Intervention: |
|
| Diuretics | |
| Clinical Impact: | Clinical studies, as well as post-marketing observations, showed that NSAIDs reduced the natriuretic effect of loop diuretics (e.g., furosemide) and thiazide diuretics in some patients. This effect has been attributed to the NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | During concomitant use of DUEXIS with diuretics, observe patients for signs of worsening renal function, in addition to assuring diuretic efficacy including antihypertensive effects [see Warnings and Precautions (5.7)]. |
| Digoxin | |
| Clinical Impact: | The concomitant use of ibuprofen with digoxin has been reported to increase the serum concentration and prolong the half-life of digoxin. |
| Intervention: | During concomitant use of DUEXIS and digoxin, monitor serum digoxin levels. |
| Lithium | |
| Clinical Impact: | NSAIDs have produced elevations of plasma lithium levels and reductions in renal lithium clearance. The mean minimum lithium concentration increased 15%, and the renal clearance decreased by approximately 20%. This effect has been attributed to NSAID inhibition of renal prostaglandin synthesis. |
| Intervention: | During concomitant use of DUEXIS and lithium, monitor patients for signs of lithium toxicity. |
| Methotrexate | |
| Clinical Impact: | Concomitant use of NSAIDs and methotrexate may increase the risk for methotrexate toxicity (e.g., neutropenia, thrombocytopenia, renal dysfunction). |
| Intervention: | During concomitant use of DUEXIS and methotrexate, monitor patients for methotrexate toxicity. |
| Cyclosporine | |
| Clinical Impact: | Concomitant use of ibuprofen and cyclosporine may increase cyclosporine's nephrotoxicity. |
| Intervention: | During concomitant use of DUEXIS and cyclosporine, monitor patients for signs of worsening renal function. |
| NSAIDs and Salicylates | |
| Clinical Impact: | Concomitant use of ibuprofen with other NSAIDs or salicylates (e.g., diflunisal, salsalate) increases the risk of GI toxicity, with little or no increase in efficacy [see Warnings and Precautions (5.2)]. |
| Intervention: | The concomitant use of DUEXIS with other NSAIDs or salicylates is not recommended. |
| Pemetrexed | |
| Clinical Impact: | Concomitant use of ibuprofen and pemetrexed may increase the risk of pemetrexed-associated myelosuppression, renal, and GI toxicity (see the pemetrexed prescribing information). |
| Intervention: | During concomitant use of DUEXIS and pemetrexed, in patients with renal impairment whose creatinine clearance ranges from 45 to 79 mL/min, monitor for myelosuppression, renal and GI toxicity. NSAIDs with short elimination half-lives (e.g., diclofenac, indomethacin) should be avoided for a period of two days before, the day of, and two days following administration of pemetrexed. In the absence of data regarding potential interaction between permetrexed and NSAIDs with longer half-lives (e.g., meloxicam, nabumetone), patients taking these NSAIDs should interrupt dosing for at least five days before, the day of, and two days following pemetrexed administration. |
| Drugs Dependent on Gastric pH for Absorption | |
| Clinical Impact | Because famotidine lowers intra-gastric acidity, this may result in reduced absorption and loss of efficacy of concomitant drugs. |
| Intervention | Concomitant administration of DUEXIS is not recommended with dasatinib, delavirdine mesylate, cefditoren, and fosamprenavir. For administration instructions of other drugs whose absorption is dependent on gastric pH, refer to their prescribing information (e.g., atazanavir, erlotinib, ketoconazole, itraconazole, nilotinib, ledipasvir/sofosbuvir, and rilpivirine). |
| Tizanidine (CYP1A2 Substrate) | |
| Clinical Impact | Famotidine is considered a weak CYP1A2 inhibitor and may lead to substantial increases in blood concentrations of tizanidine, a CYP1A2 substrate. |
| Intervention | Avoid concomitant use with DUEXIS. If concomitant use is necessary, monitor for hypotension, bradycardia or excessive drowsiness. Refer to the full prescribing information for tizanidine. |
8. Use In Specific Populations
8.1 Pregnancy
Data
Human Data
When used to delay preterm labor, inhibitors of prostaglandin synthesis, including NSAIDs such ibuprofen, may increase the risk of neonatal complications such as necrotizing enterocolitis, patent ductus arteriosus and intracranial hemorrhage. Ibuprofen treatment given in late pregnancy to delay parturition has been associated with persistent pulmonary hypertension, renal dysfunction and abnormal prostaglandin E levels in preterm infants.
8.4 Pediatric Use
Safety and effectiveness of DUEXIS in pediatric patients have not been established.
8.5 Geriatric Use
Elderly patients, compared to younger patients, are at greater risk for NSAID-associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, start dosing at the low end of the dosing range, and monitor patients for adverse effects [see Warnings and Precautions (5.1, 5.2, 5.4, 5.7, 5.16)].
The clinical trials primarily enrolled patients less than 65 years of age. Of the 1022 patients in clinical studies of DUEXIS, 18% (249 patients) were 65 years of age or older. Efficacy results in patients who are greater than or equal to 65 years of age are summarized in the CLINICAL STUDIES section [see Clinical Studies (14)].
Famotidine is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and adjusting dose interval, and it may be useful to monitor renal function [see Warnings and Precautions (5.7)].
8.6 Renal Insufficiency
In adult patients with renal insufficiency (creatinine clearance < 50 mL/min), the elimination half-life of famotidine is increased. Since CNS adverse effects have been reported in patients with creatinine clearance < 50 mL/min and the dosage of the famotidine component in DUEXIS is fixed, DUEXIS is not recommended in these patients [see Warnings and Precautions (5.7)].
10. Overdosage
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare [see Warnings and Precautions (5.1, 5.2, 5.5, 5.7, 5.9)].
No data are available with regard to overdose of DUEXIS. Findings related to the individual active substances are listed below.
11. Duexis Description
DUEXIS (ibuprofen and famotidine) is supplied as a tablet for oral administration which combines the nonsteroidal anti- inflammatory drug, ibuprofen, and the histamine H2-receptor antagonist, famotidine.
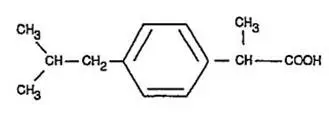
Ibuprofen is (±)-2-(p-isobutylphenyl)propionic acid. Its chemical formula is C13H18O2 and molecular weight is 206.28. Ibuprofen is a white powder that is very slightly soluble in water (<1 mg/mL) and readily soluble in organic solvents such as ethanol and acetone. Its structural formula is:
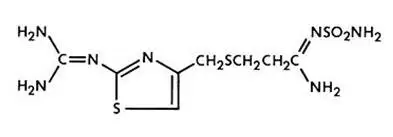
Famotidine is N'-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propanimidamide. Its chemical formula is C8H15N7O2S3 and molecular weight is 337.45. Famotidine is a white to pale yellow crystalline compound that is freely soluble in glacial acetic acid, slightly soluble in methanol, very slightly soluble in water, and practically insoluble in ethanol. Its structural formula is:
Each DUEXIS tablet contains ibuprofen, USP (800 mg) and famotidine, USP (26.6 mg). The inactive ingredients in DUEXIS include: microcrystalline cellulose, anhydrous lactose, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, purified water, povidone, titanium dioxide, polyethylene glycol, polysorbate 80, polyvinyl alcohol, hypromellose, talc, FD&C Blue #2/Indigo Carmine Aluminum Lake, and FD&C Blue #1/Brilliant Blue FCF Aluminum Lake.
12. Duexis - Clinical Pharmacology
12.1 Mechanism of Action
DUEXIS is a fixed-combination tablet of ibuprofen and famotidine. The ibuprofen component has analgesic, anti- inflammatory, and antipyretic properties. The mechanism of action of the ibuprofen component of DUEXIS, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Ibuprofen is a potent inhibitor of prostaglandin synthesis in vitro. Ibuprofen concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because ibuprofen is an inhibitor of prostaglandin synthesis, its mode of action may be due to an increase of prostaglandins in peripheral tissues.
Famotidine is a competitive inhibitor of histamine H2-receptors. The primary clinically important pharmacologic activity of famotidine is inhibition of gastric secretion. Both the acid concentration and volume of gastric secretion are suppressed by famotidine, while changes in pepsin secretion are proportional to volume output.
Systemic effects of famotidine in the CNS, cardiovascular, respiratory, or endocrine systems were not noted in clinical pharmacology studies. Also, no antiandrogenic effects were noted. Serum hormone levels, including prolactin, cortisol, thyroxine (T4), and testosterone, were not altered after treatment with famotidine.
12.2 Pharmacodynamics
In a healthy volunteer study, ibuprofen 400 mg given once daily, administered 2 hours prior to immediate-release aspirin (81 mg) for 6 days, showed an interaction with the antiplatelet activity of aspirin as measured by % serum thromboxane B2 (TxB2) inhibition at 24 hours following the day-6 aspirin dose [53%]. An interaction was still observed, but minimized, when ibuprofen 400 mg given once-daily was administered as early as 8 hours prior to the immediate-release aspirin dose [90.7%]. However, there was no interaction with the antiplatelet activity of aspirin when ibuprofen 400 mg, given once daily, was administered 2 hours after (but not concomitantly, 15 min, or 30 min after) the immediate-release aspirin dose [99.2%].
In another study, where immediate-release aspirin 81 mg was administered once daily with ibuprofen 400 mg given three times daily (1, 7, and 13 hours post-aspirin dose) for 10 consecutive days, the mean % serum thromboxane B2 (TxB2) inhibition suggested no interaction with the antiplatelet activity of aspirin [98.3%]. However, there were individual subjects with serum TxB2 inhibition below 95%, with the lowest being 90.2%.
When a similarly designed study was conducted with enteric-coated aspirin, where healthy subjects were administered enteric-coated aspirin 81 mg once daily for 6 days and ibuprofen 400 mg three times daily (2, 7, and 12 h post-aspirin dose) for 6 days, there was an interaction with the antiplatelet activity at 24 hours following the day-6 aspirin dose [67%] [see Drug Interactions (7)].
14. Clinical Studies
Two multicenter, double-blind, active-controlled, randomized, 24-week studies of DUEXIS were conducted in patients who were expected to require daily administration of an NSAID for at least the coming 6 months for conditions such as the following: osteoarthritis, rheumatoid arthritis, chronic low back pain, chronic regional pain syndrome, and chronic soft tissue pain. Patients were assigned randomly, in approximately a 2:1 ratio, to treatment with either DUEXIS or ibuprofen (800 mg) three times a day for 24 consecutive weeks. A total of 1533 patients were enrolled and ranged in age from 39 to 80 years (median age 55 years) with 68% females. Race was distributed as follows: 79% Caucasian, 18% African-American, and 3% Other. Approximately 15% of the patients in Studies 301 and 303 were taking concurrent low-dose aspirin (less than or equal to 325 mg daily), 18% were 65 years of age or older, and 6% had a history of previous upper gastrointestinal ulcer. Although H. pylori status was negative at baseline, H. pylori status was not reassessed during the trials.
Studies 301 and 303 compared the incidence of upper gastrointestinal (gastric and/or duodenal) ulcer formation in a total 930 patients taking DUEXIS (ibuprofen and famotidine) and 452 patients taking ibuprofen only, either as a primary or secondary endpoint. In both trials, DUEXIS was associated with a statistically significantly reduction in the risk of developing upper gastrointestinal ulcers compared to taking ibuprofen only during the 6 month study period. The data are presented below in Tables 4 and 5. Two analyses for each endpoint were conducted. In one analysis patients who terminated early, without an endoscopic evaluation within 14 days of their last dose of study drug, were classified as not having an ulcer. In the second analysis, those patients were classified as having an ulcer. Both analyses exclude patients who terminated study prior to the first scheduled endoscopy at 8 weeks.
| DUEXIS % (n/N) | Ibuprofen % (n/N) | P-value* | |
|---|---|---|---|
|
|||
| Primary endpoint | |||
| Upper gastrointestinal ulcer† | 10.5% (40/380) | 20.0% (38/190) | 0.002 |
| Upper gastrointestinal ulcer‡ | 22.9% (87/380) | 32.1% (61/190) | 0.020 |
| Secondary endpoint | |||
| Gastric ulcer† | 9.7% (37/380) | 17.9% (34/190) | 0.005 |
| Gastric ulcer‡ | 22.4% (85/380) | 30.0% (57/190) | 0.052 |
| DUEXIS % (n/N) | Ibuprofen % (n/N) | P-value* | |
|---|---|---|---|
|
|||
| Primary endpoint | |||
| Gastric ulcer† | 8.7% (39/447) | 17.6% (38/216) | 0.0004 |
| Gastric ulcer‡ | 17.4% (78/447) | 31.0% (67/216) | <0.0001 |
| Secondary endpoint | |||
| Upper gastrointestinal ulcer† | 10.1% (45/447) | 21.3% (46/216) | <0.0001 |
| Upper gastrointestinal ulcer‡ | 18.6% (83/447) | 34.3% (74/216) | <0.0001 |
Subgroup analyses of patients who used low-dose aspirin (less than or equal to 325 mg daily), were 65 years and older, or had a prior history of gastrointestinal ulcer are summarized as follows:
Of the 1022 patients in clinical studies of DUEXIS, 15% (213 patients) used low-dose aspirin and the results were consistent with the overall findings of the study. In these clinical studies 16% of patients who used low-dose aspirin who were treated with DUEXIS developed an upper gastrointestinal ulcer compared to 35% of those patients who received only ibuprofen.
The clinical trials primarily enrolled patients less than 65 years without a prior history of gastrointestinal ulcer. Of the 1022 patients in clinical studies of DUEXIS, 18% (249 patients) were 65 years of age or older. In these clinical studies, 23% of patients 65 years of age and older who were treated with DUEXIS developed an upper gastrointestinal ulcer compared to 27% of those patients who received only ibuprofen [see Use in Specific Populations (8.5)].
Of the 1022 patients in clinical studies of DUEXIS, 6% had a prior history of gastrointestinal ulcer. In these clinical studies, 25% of patients with a prior history of gastrointestinal ulcer who were treated with DUEXIS developed an upper gastrointestinal ulcer compared to 24% of those patients who received only ibuprofen.
16. How is Duexis supplied
DUEXIS (ibuprofen and famotidine) tablets, 800 mg/26.6 mg, are light blue, oval, biconvex, film-coated tablets debossed with "HZT" on one side and supplied as:
| NDC Number | Size |
| 75987-010-03 | Bottle of 90 tablets |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Inform patients, families, or caregivers of the following before initiating therapy with DUEXIS and periodically during the course of ongoing therapy.
| Medication Guide DUEXIS (dew-EX-iss) (ibuprofen and famotidine) tablets, for oral use |
||||
|---|---|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 4/2021 DUE-US-MG-001 |
|||
| What is the most important information I should know about DUEXIS? DUEXIS can cause serious side effects including:
|
||||
|
|
|
||
|
||||
|
|
|||
| You should take DUEXIS exactly as prescribed, at the lowest dose possible and for the shortest time needed. DUEXIS contains a non-steroidal anti-inflammatory drug NSAID (ibuprofen). Do not use DUEXIS with other medicines to lessen pain or fever or with other medicines for colds or sleeping problems without talking to your healthcare provider first, because they may contain an NSAID also. DUEXIS may help your acid-related symptoms, but you could still have serious stomach problems. Talk with your healthcare provider. DUEXIS contains ibuprofen, an NSAID and famotidine, a histamine H2-receptor blocker medicine. |
||||
| What is DUEXIS?
DUEXIS is a prescription medicine used to:
|
||||
Do not take DUEXIS:
|
||||
Before taking DUEXIS, tell your healthcare provider about all of your medical conditions, including if you:
|
||||
How should I take DUEXIS?
|
||||
| What are the possible side effects of DUEXIS? DUEXIS can cause serious side effects, including: See "What is the most important information I should know about DUEXIS? |
||||
|
|
|||
| Other side effects of DUEXIS include: stomach pain, constipation, diarrhea, gas, heartburn, nausea, vomiting, and dizziness. Get emergency help right away if you get any of the following symptoms: |
||||
|
|
|||
| Stop taking DUEXIS and call your healthcare provider right away if you get any of the following symptoms: | ||||
|
|
|||
| If you take too much DUEXIS, call your poison control center at 1-800-222-1222. These are not all the possible side effects of DUEXIS. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
Other information about NSAIDs
|
||||
| General information about the safe and effective use of DUEXIS
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use DUEXIS for a condition for which it was not prescribed. Do not give DUEXIS to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals. |
||||
| What are the ingredients in DUEXIS? Active ingredients: ibuprofen and famotidine Inactive ingredients: microcrystalline cellulose, anhydrous lactose, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, purified water, povidone, titanium dioxide, polyethylene glycol, polysorbate 80, polyvinyl alcohol, hypromellose, talc, FD&C Blue#2/Indigo Carmine Aluminum Lake, and FD&C Blue #1/Brilliant Blue FCF Aluminum Lake. Distributed by: Horizon Medicines LLC., Deerfield, IL 60015 For more information, go to www.DUEXIS.com or call 1-866-479-6742. |
||||
| DUEXIS
ibuprofen and famotidine tablet, coated |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Horizon Therapeutics USA, Inc. (033470838) |





