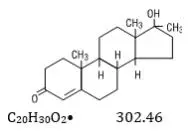
Drug Class: Sex hormone combinations
WARNINGS
1. ESTROGENS HAVE BEEN REPORTED TO INCREASE THE RISK OF ENDOMETRIAL CARCINOMA
Three independent case control studies have reported an increased risk of endometrial cancer in postmenopausal women exposed to exogenous estrogens for prolonged periods.1-3 This risk was independent of the other known risk factors for endometrial cancer. These studies are further supported by the finding that incidence rates of endometrial cancer have increased sharply since 1969 in eight different areas of the United States with population-based cancer reporting systems, an increase which may be related to the rapidly expanding use of estrogens during the last decade. 4
The three case control studies reported that the risk of endometrial cancer in estrogen users was about 4.5 to 13.9 times greater than in nonusers. The risk appears to depend on both duration of treatment1 and on estrogen dose. 3 In view of these findings, when estrogens are used for the treatment of menopausal symptoms, the lowest dose that will control symptoms should be utilized and medication should be discontinued as soon as possible. When prolonged treatment is medically indicated, the patient should be reassessed on at least a semiannual basis to determine the need for continued therapy. Although the evidence must be considered preliminary, one study suggests that cyclic administration of low doses of estrogen may carry less risk than continuous administration, 3 it therefore appears prudent to utilize such a regimen.
Close clinical surveillance of all women taking estrogens is important. In all cases of undiagnosed persistent or recurring abnormal vaginal bleeding, adequate diagnostic measures should be undertaken to rule out malignancy.
There is no evidence at present that "natural" estrogens are more or less hazardous than "synthetic" estrogens at equiestrogenic doses.
2. ESTROGENS SHOULD NOT BE USED DURING PREGNANCY
The use of female sex hormones, both estrogens and progestogens, during early pregnancy may seriously damage the offspring. It has been shown that females exposed in utero to diethylstilbestrol, a non-steroidal estrogen, have an increased risk of developing in later life a form of vaginal or cervical cancer that is ordinarily extremely rare. 5,6 This risk has been estimated as not greater than 4 per 1000 exposures. 7 Furthermore, a high percentage of such exposed women (from 30 to 90 percent) have been found to have vaginal adenosis, 8-12 epithelial changes of the vagina and cervix. Although these changes are histologically benign, it is not known whether they are precursors of malignancy. Although similar data are not available with the use of other estrogens, it cannot be presumed they would not induce similar changes.
Several reports suggest an association between intrauterine exposure to female sex hormones and congenital anomalies, including congenital heart defects and limb reduction defects.13-16 One case control study16 estimated a 4.7 fold increased risk of limb reduction defects in infants exposed in utero to sex hormones (oral contraceptives, hormone withdrawal tests for pregnancy, or attempted treatment for threatened abortion). Some of these exposures were very short and involved only a few days of treatment. The data suggest that the risk of limb reduction defects in exposed fetuses is somewhat less than 1 per 1000.
In the past, female sex hormones have been used during pregnancy in an attempt to treat threatened or habitual abortion. There is considerable evidence that estrogens are ineffective for these indications, and there is no evidence from well controlled studies that progesterones are effective for these uses.
IF ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE FULL STRENGTH or ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE HALF STRENGTH is used during pregnancy, or if the patient becomes pregnant while taking this drug, she should be apprised of the potential risks to the fetus, and the advisability of pregnancy continuation.
CONTRAINDICATIONS
Estrogens should not be used in women with any of the following conditions:
1. Known or suspected cancer of the breast except in appropriately selected patients being treated for metastatic disease.
2. Known or suspected estrogen-dependent neoplasia.
3. Known or suspected pregnancy (See Boxed Warning).
4. Undiagnosed abnormal genital bleeding.
5. Active thrombophlebitis or thromboembolic disorders.
6. A past history of thrombophlebitis, thrombosis, or thromboembolic disorders associated with previous estrogen use (except when in treatment of breast malignancy).
Methyltestosterone should not be used in:
1. The presence of severe liver damage.
2. Pregnancy and in breast-feeding mothers because of the possibility of masculinization of the female fetus or breast-fed infant.
Warnings
Associated with Estrogens
- Induction of malignant neoplasms. Long term continuous administration of natural and synthetic estrogens in certain animal species increases this frequency of carcinomas of the breast, cervix, vagina, and liver. There is now evidence that estrogens increase the risk of carcinoma of the endometrium in humans (See Boxed Warning).
At the present time there is no satisfactory evidence that estrogens given to postmenopausal women increase the risk of cancer of the breast, 18 although a recent long-term follow-up of a single physician's practice has raised this possibility. 18a Because of the animal data, there is a need for caution in prescribing estrogens for women with a strong family history of breast cancer or who have breast nodules, fibrocystic disease, or abnormal mammograms.
2. Gallbladder disease. A recent study has reported a 2 to 3-fold increase in the risk of surgically confirmed gallbladder disease in women receiving postmenopausal estrogens, 18 similar to the 2-fold increase previously noted in users of oral contraceptives. 19-24a In the case of oral contraceptives the increased risk appeared after two years of use. 24
3. Effects similar to those caused by estrogen-progesterone oral contraceptives. There are several serious adverse effects of oral contraceptives, most of which have not, up to now, been documented as consequences of postmenopausal estrogen therapy. This may reflect the comparatively low doses of estrogen used in postmenopausal women. It would be expected that the larger doses of estrogen used to treat prostatic or breast cancer or postpartum breast engorgement are more likely to result in these adverse effects, and, in fact, it has been shown that there is an increased risk of thrombosis in men receiving estrogens for prostatic cancer and women for postpartum breast engorgement. 20-23
a. Thromboembolic disease. It is now well established that users of oral contraceptives have an increased risk of various thromboembolic and thrombotic vascular diseases, such as thrombophlebitis, pulmonary embolism, stroke, and myocardial infarction. 24-31 Cases of retinal thrombosis, mesenteric thrombosis, and optic neuritis have been reported in oral contraceptive users. There is evidence that the risk of several of these adverse reactions is related to the dose of the drug. 32-33 An increased risk of postsurgery thromboembolic complications has also been reported in users of oral contraceptives. 34-35 If feasible, estrogen should be discontinued at least 4 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
While an increased rate of thromboembolic and thrombotic disease in postmenopausal users of estrogens has not been found, 18-36 this does not rule out the possibility that such an increase may be present or that subgroups of women who have underlying risk factors or who are receiving relatively large doses of estrogens may have increased risk. Therefore estrogens should not be used in persons with active thrombophlebitis or thromboembolic disorders, and they should not be used (except in treatment of malignancy) in persons with a history of such disorders in association with estrogen use. They should be used with caution in patients with cerebral vascular or coronary artery disease and only for those in whom estrogens are clearly needed.
Large doses of estrogen (5 mg esterified estrogens per day), comparable to those used to treat cancer of the prostate and breast, have been shown in a large prospective clinical trial in men
37 to increase the risk of nonfatal myocardial infarction, pulmonary embolism and thrombophlebitis. When estrogen doses of this size are used, any of the thromboembolic and thrombotic adverse effects associated with oral contraceptive use should be considered a clear risk.
b.
Hepatic adenoma. Benign hepatic adenomas appear to be associated with the use of oral contraceptives.
38-40 Although benign and rare, these may rupture and may cause death through intra-abdominal hemorrhage. Such lesions have not yet been reported in association with other estrogen or progestogen preparations but should be considered in estrogen users having abdominal pain and tenderness, abdominal mass, or hypovolemic shock. Hepatocellular carcinoma has also been reported in women taking estrogen-containing oral contraceptives.
39 The relationship of this malignancy to these drugs is not known at this time.
c. Elevated blood pressure. Increased blood pressure is not uncommon in women using oral contraceptives. There is now a report that this may occur with use of estrogens in the menopause 41 and blood pressure should be monitored with estrogen use, especially if high doses are used.
d. Glucose tolerance. A worsening of glucose tolerance has been observed in a significant percentage of patients of estrogen-containing oral contraceptives. For this reason, diabetic patients should be carefully observed while receiving estrogens.
4. Hypercalcemia. Administration of estrogens may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If this occurs, the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Associated with Methyltestosterone
In patients with breast cancer, androgen therapy may cause hypercalcemia by stimulating osteolysis. In this case the drug should be discontinued.
Prolonged use of high doses of androgens has been associated with the development of peliosis hepatis and hepatic neoplasms including hepatocellular carcinoma. (See PRECAUTIONS – Carcinogenesis).
Peliosis hepatis can be a life-threatening or fatal complication.
Cholestatic hepatitis and jaundice occur with 17-alpha-alkyl androgens at a relatively low dose. If cholestatic hepatitis with jaundice appears or if liver function tests become abnormal, the androgen should be discontinued and the etiology should be determined. Drug-induced jaundice is reversible when the medication is discontinued.
Edema with or without heart failure may be a serious complication in patients with preexisting cardiac, renal, or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required.
Precautions
Associated with Estrogens
A. General Precautions
1. A complete medical and family history should be taken prior to the initiation of any estrogen therapy. The pretreatment and periodic physical examinations should include special reference to blood pressure, breasts, abdomen, and pelvic organs, and should include a Papanicolaou smear. As a general rule, estrogen should not be prescribed for longer than one year without another physical examination being performed.
2. Fluid retention– Because estrogens may cause some degree of fluid retention, conditions which might be influenced by this factor such as asthma, epilepsy, migraine, and cardiac or renal dysfunction, require careful observation.
3. Certain patients may develop undesirable manifestations of excessive estrogenic stimulation, such as abnormal or excessive uterine bleeding, mastodynia, etc.
4. Oral contraceptives appear to be associated with an increased incidence of mental depression.24 Although it is not clear whether this is due to the estrogenic or progestogenic component of the contraceptive, patients with a history of depression should be carefully observed.
5. Preexisting uterine leiomyomata may increase in size during estrogen use.
6. The pathologist should be advised of estrogen therapy when relevant specimens are submitted.
7. Patients with a past history of jaundice during pregnancy have an increased risk of recurrence of jaundice while receiving estrogen-containing oral contraceptive therapy. If jaundice develops in any patient receiving estrogen, the medication should be discontinued while the cause is investigated.
8. Estrogens may be poorly metabolized in patients with impaired liver function and they should be administered with caution in such patients.
9. Because estrogens influence the metabolism of calcium and phosphorus, they should be used with caution in patients with metabolic bone diseases that are associated with hypercalcemia or in patients with renal insufficiency.
10. Because of the effects of estrogens on epiphyseal closure, they should be used judiciously in young patients in whom bone growth is not complete.
11.
Certain endocrine and liver function tests may be affected by estrogen-containing oral contraceptives. The following similar changes may be expected with larger doses of estrogen:
a.
Increased sulfobromophthalein retention.
b.
Increased prothrombin and factors VII, VIII, IX and X; decreased antithrombin 3: increased norepinephrine induced platelet aggregability.
c.
Increased thyroid binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by PBI, T4 by column, or T4 by radioimmunoassay. Free T3 resin uptakes is decreased, reflecting the elevated TBG; free T4 concentration is unaltered.
d.
Impaired glucose tolerance.
e.
Decreased pregnanediol excretion.
f.
Reduced response to metyrapone test.
g.
Reduced serum folate concentration.
h.
Increased serum triglyceride and phospholipid concentration.
B. Information for the Patient
See text of Patient Package Insert which appears after the REFERENCES.
C. Pregnancy Category X
See CONTRAINDICATIONS and Boxed WARNING.
D. Nursing Mothers
As a general principle, the administration of any drug to nursing mothers should be done only when clearly necessary since many drugs are excreted in human milk.
Associated with Methyltestosterone
F. Carcinogenesis
Animal Data. Testosterone has been tested by subcutaneous injection and implantation in mice and rats. The implant induced cervical-uterine tumors in mice, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
Human Data. There are rare reports of hepatocellular carcinoma in patients receiving long-term therapy with androgens in high doses. Withdrawal of the drugs did not lead to regression of the tumors in all cases.
Geriatric Patients treated with androgens may be at an increased risk for the development of prostatic hypertrophy and prostatic carcinoma.
| ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE
esterified estrogens, methyltestosterone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE
esterified estrogens, methyltestosterone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Syntho Pharmaceuticals, Inc. (088797407) |
| Registrant - Syntho Pharmaceuticals, Inc. (088797407) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Syntho Pharmaceuticals, Inc. | 088797407 | manufacture(66576-283, 66576-284) | |