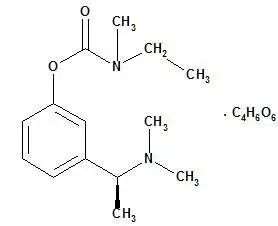
Drug Detail:Exelon (Rivastigmine (oral) [ ri-va-stig-meen ])
Drug Class: Cholinesterase inhibitors
Highlights of Prescribing Information
EXELON® (rivastigmine tartrate) capsules, for oral use
EXELON® (rivastigmine tartrate) oral solution
Initial U.S. Approval: 2000
Indications and Usage for Exelon
EXELON is an acetylcholinesterase inhibitor indicated for treatment of:
- Mild-to-moderate dementia of the Alzheimer’s type (AD) (1.1)
- Mild-to-moderate dementia associated with Parkinson’s disease (PD) (1.2)
Exelon Dosage and Administration
Alzheimer’s Disease (2.1):
- Initial Dose: Initiate treatment with 1.5 mg twice a day.
- Dose Titration: After a minimum of 2 weeks, if tolerated, increase dose to 3 mg twice a day and further to 4.5 mg twice a day and 6 mg twice a day if tolerated with a minimum of 2 weeks at each dose
Parkinson’s Disease Dementia (PDD) (2.2):
- Initial Dose: Initiate treatment with 1.5 mg twice a day.
- Dose Titration: After a minimum of 4 weeks, if tolerated, increase dose to 3 mg twice a day and further to 4.5 mg twice a day and 6 mg twice a day if tolerated with a minimum of 4 weeks at each dose.
EXELON should be taken with meals in divided doses in the morning and evening (2.1, 2.2). EXELON Oral Solution and EXELON Capsules may be interchanged at equal doses (2.5)
Dosage Forms and Strengths
- Capsules: 1.5 mg, 3 mg, 4.5 mg, or 6 mg (3.1)
- Oral Solution: 2 mg/mL (3.2)
Contraindications
- Known hypersensitivity to rivastigmine, other carbamate derivatives or other components of the formulation. (4)
- History of application site reaction with rivastigmine transdermal patch suggestive of allergic contact dermatitis, in the absence of negative allergy testing. (4, 5.2)
Warnings and Precautions
- Gastrointestinal adverse reactions may include significant nausea, vomiting, diarrhea, anorexia/decreased appetite, and weight loss, and may necessitate treatment interruption. Dehydration may result from prolonged vomiting or diarrhea and can be associated with serious outcomes. (5.1)
- Discontinue rivastigmine in case of disseminated allergic dermatitis, which may occur after oral or transdermal administration (4, 5.2). In patients with suspected allergic contact dermatitis after transdermal rivastigmine use, switch to oral rivastigmine only after negative allergy testing.
Adverse Reactions/Side Effects
Most common adverse reactions (greater than 5% and 2 times greater than placebo): nausea, vomiting, anorexia, dyspepsia, and asthenia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Concomitant use with metoclopramide, beta-blockers, or cholinomimetic and anticholinergic drugs is not recommended (7.1, 7.2, 7.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2018
Full Prescribing Information
1. Indications and Usage for Exelon
2. Exelon Dosage and Administration
2.1 Dosing in Alzheimer’s Disease
EXELON should be taken with meals in divided doses in the morning and evening.
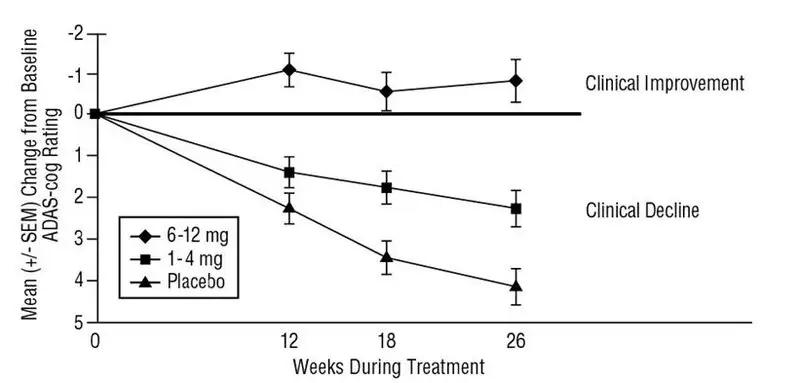
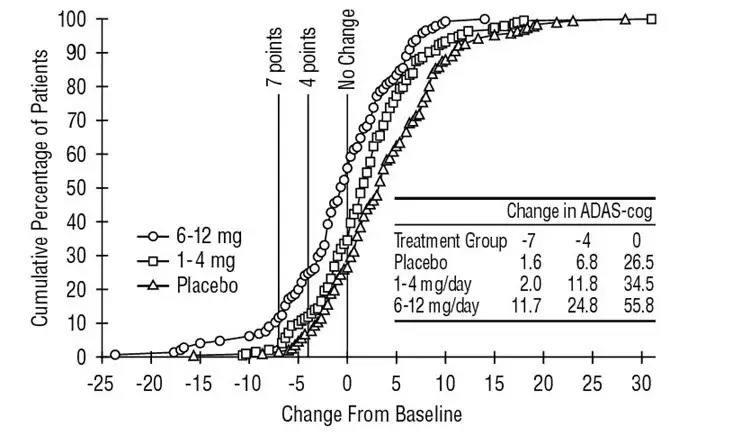
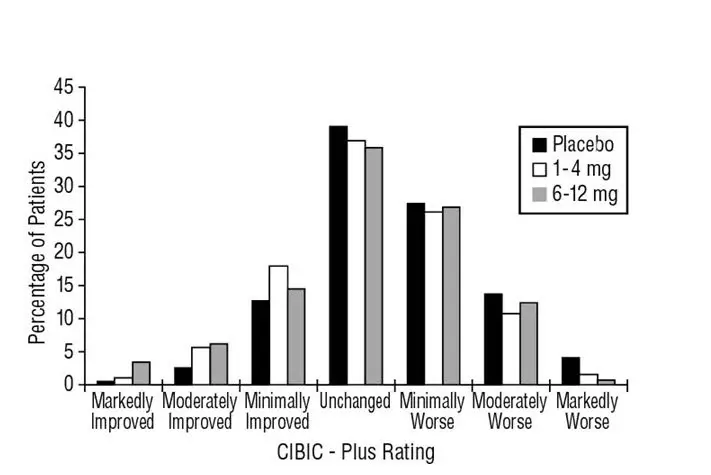
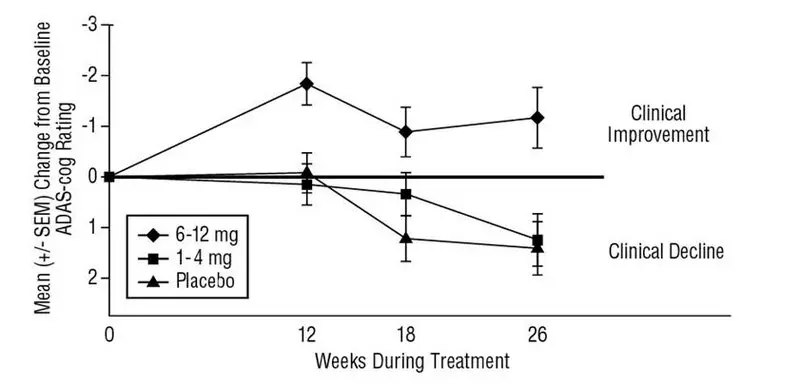
The recommended dosage of EXELON Oral Solution and EXELON Capsules in Alzheimer’s disease (AD) is 6 mg to 12 mg per day, administered twice a day (daily doses of 3 mg to 6 mg twice a day). There is evidence from the clinical trials that doses at the higher end of this range may be more beneficial.
Initial Dose
Initiate treatment with the 1.5 mg twice a day with EXELON.
Dose Titration
After a minimum of 2 weeks and if well tolerated, increase the dose to 3 mg twice a day. Subsequent increases to 4.5 mg twice a day and 6 mg twice a day should be attempted after a minimum of 2 weeks at the previous dose and if well tolerated. The maximum dose is 6 mg twice a day (12 mg per day).
2.2 Dosing in Parkinson’s Disease Dementia
EXELON should be taken with meals in divided doses in the morning and evening.
The dosage of EXELON shown to be effective in the single controlled clinical trial conducted in dementia associated with Parkinson’s disease is 3 mg to 12 mg per day, administered twice a day (daily doses of 1.5 mg to 6 mg twice a day).
Initial Dose
Initiate treatment with the 1.5 mg twice a day with EXELON.
Dose Titration
After a minimum of 4 weeks and if well tolerated, increase the dose to 3 mg twice a day. Subsequent increases to 4.5 mg twice a day and 6 mg twice a day should be attempted after a minimum of 4 weeks at the previous dose and if well tolerated. The maximum dose is 6 mg twice a day (12 mg per day).
2.3 Interruption of Treatment
If adverse effects (e.g., nausea, vomiting, abdominal pain, loss of appetite) cause intolerance during treatment, the patient should be instructed to discontinue treatment for several doses and then restart at the same or next lower dose level.
If dosing is interrupted for 3 days or fewer, restart treatment with the same or lower dose of EXELON. If dosing is interrupted for more than 3 days, treatment should be restarted with 1.5 mg twice a day and titrated as described above [see Warnings and Precautions (5.1)].
2.4 Dosing in Specific Populations
Dosing Modifications in Patients with Renal Impairment
Patients with moderate and severe renal impairment may be able to only tolerate lower doses.
Dosing Modifications in Patients with Hepatic Impairment
Patients with mild (Child-Pugh score 5 to 6) and moderate (Child-Pugh score 7 to 9) hepatic impairment may be able to only tolerate lower doses. No data are available on the use of rivastigmine in patients with severe hepatic impairment.
Dosing Modifications in Patients with Low Body Weight
Carefully titrate and monitor patients with low body weight (less than 50 kg) for toxicities (e.g., excessive nausea, vomiting), and consider reducing the dose if such toxicities develop.
2.5 Important Administration Instructions
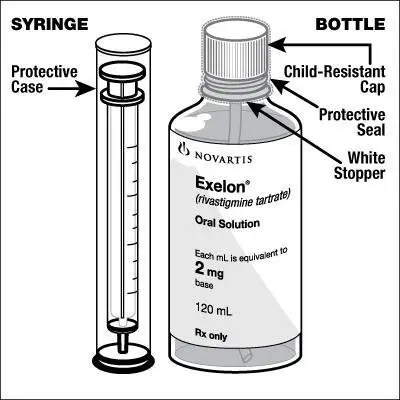
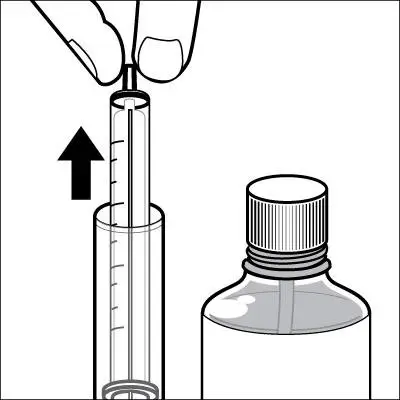
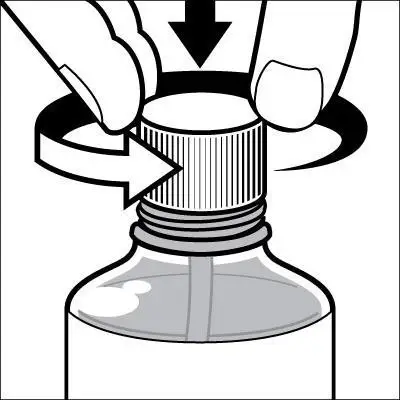
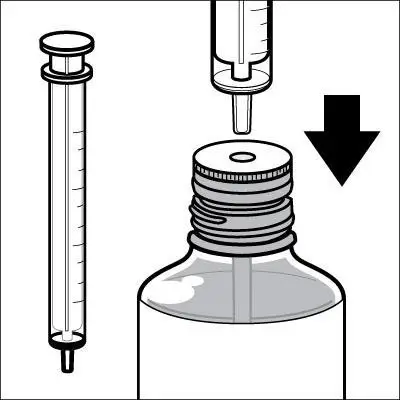
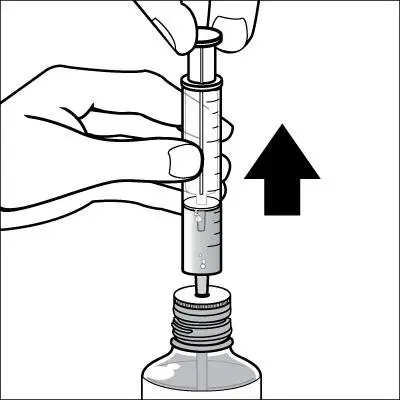
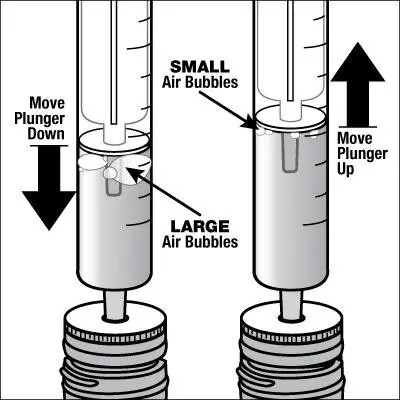
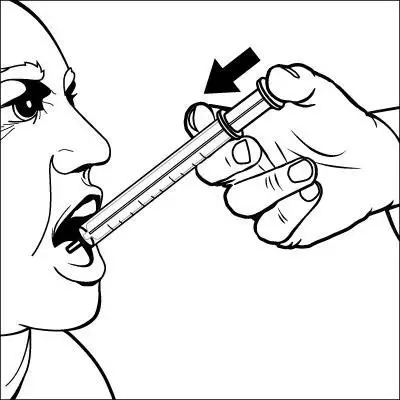
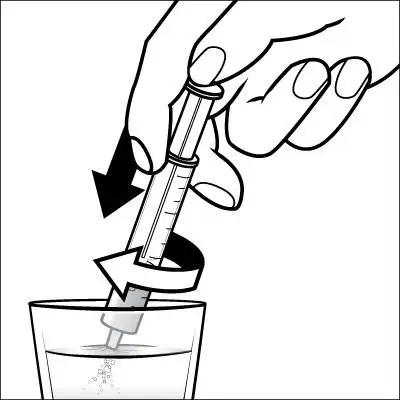
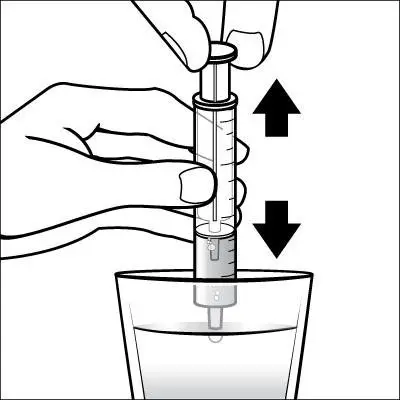
Caregivers should be instructed in the correct procedure for administering EXELON Oral Solution. In addition, they should be directed to the Instruction Sheet (included with the product) describing how the solution is to be administered. Caregivers should direct questions about the administration of the solution to either their physician or pharmacist [see Patient Counseling Information (17)].
Patients should be instructed to remove the oral dosing syringe provided in its protective case, and using the provided syringe, withdraw the prescribed amount of EXELON Oral Solution from the container. Each dose of EXELON Oral Solution may be swallowed directly from the syringe or first mixed with a small glass of water, cold fruit juice, or soda. Patients should be instructed to stir and drink the mixture.
EXELON Oral Solution and EXELON Capsules may be interchanged at equal doses.
3. Dosage Forms and Strengths
3.1 EXELON Capsules
Capsules, containing rivastigmine tartrate equivalent to 1.5 mg, 3 mg, 4.5 mg, or 6 mg of rivastigmine base, are available as follows:
- 1.5 mg capsule – yellow, “EXELON 1.5 mg” is printed in red on the body of the capsule
- 3 mg capsule – orange, “EXELON 3 mg” is printed in red on the body of the capsule
- 4.5 mg capsule – red, “EXELON 4.5 mg” is printed in white on the body of the capsule
- 6 mg capsule – orange and red, “EXELON 6 mg” is printed in red on the body of the capsule
4. Contraindications
EXELON is contraindicated in patients with:
- known hypersensitivity to rivastigmine, other carbamate derivatives or other components of the formulation [see Description (11)]
- a previous history of application site reaction with rivastigmine transdermal patch suggestive of allergic contact dermatitis, in the absence of negative allergy testing [see Warnings and Precautions (5.2)]
Isolated cases of generalized skin reactions have been described in postmarketing experience [see Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Gastrointestinal Adverse Reactions
EXELON can cause gastrointestinal adverse reactions, including significant nausea, vomiting, diarrhea, anorexia/decreased appetite, and weight loss. Dehydration may result from prolonged vomiting or diarrhea and can be associated with serious outcomes. The incidence and severity of these reactions are dose-related [see Adverse Reactions (6.1)]. For this reason, patients should always be started at a dose of 1.5 mg twice a day and titrated to their maintenance dose.
If treatment is interrupted for longer than 3 days, treatment should be reinitiated with the lowest daily dose [see Dosage and Administration (2.3)] to reduce the possibility of severe vomiting and its potentially serious sequelae (e.g., there has been one postmarketing report of severe vomiting with esophageal rupture following inappropriate reinitiation of treatment with a 4.5-mg dose after 8 weeks of treatment interruption).
Inform caregivers to monitor for gastrointestinal adverse reactions and to inform the physician if they occur. It is critical to inform caregivers that if therapy has been interrupted for more than 3 days because of intolerance, the next dose should not be administered without contacting the physician regarding proper retitration.
5.2 Allergic Dermatitis
There have been isolated postmarketing reports of patients experiencing disseminated allergic dermatitis when administered rivastigmine irrespective of the route of administration (oral or transdermal). Treatment should be discontinued if disseminated allergic dermatitis occurs [see Contraindications (4)]. Patients and caregivers should be instructed accordingly [see Patient Counseling Information (17)].
In patients who develop application site reactions, suggestive of allergic contact dermatitis to EXELON PATCH, and who still require rivastigmine, treatment should be switched to oral rivastigmine only after negative allergy testing and under close medical supervision. It is possible that some patients sensitized to rivastigmine by exposure to rivastigmine patch may not be able to take rivastigmine in any form.
6. Adverse Reactions/Side Effects
The following adverse reactions are described below and elsewhere in the labeling:
- Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.1)]
- Allergic Dermatitis [see Warnings and Precautions (5.2)]
- Other Adverse Reactions from Increased Cholinergic Activity [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
EXELON has been administered to over 5,297 individuals during clinical trials worldwide. Of these, 4,326 patients have been treated for at least 3 months, 3,407 patients have been treated for at least 6 months, 2,150 patients have been treated for 1 year, 1,250 patients have been treated for 2 years, and 168 patients have been treated for over 3 years. With regard to exposure to the highest dose, 2,809 patients were exposed to doses of 10 mg to 12 mg, 2,615 patients treated for 3 months, 2,328 patients treated for 6 months, 1,378 patients treated for 1 year, 917 patients treated for 2 years, and 129 patients treated for over 3 years.
Mild-to-Moderate Alzheimer’s Disease
Most Common Adverse Reactions
The most common adverse reactions, defined as those occurring at a frequency of at least 5% and twice the placebo rate, are largely predicted by EXELON's cholinergic effects. These include nausea, vomiting, anorexia, dyspepsia, and asthenia.
Gastrointestinal Adverse Reactions
EXELON use is associated with significant nausea, vomiting, and weight loss [see Warnings and Precautions (5.1)].
Discontinuation Rates
The rate of discontinuation due to adverse events in controlled clinical trials of EXELON (rivastigmine tartrate) was 15% for patients receiving 6 mg to 12 mg per day compared to 5% for patients on placebo during forced weekly dose titration. While on a maintenance dose, the rates were 6% for patients on EXELON compared to 4% for those on placebo.
The most common adverse reactions leading to discontinuation, defined as those occurring in at least 2% of patients and at twice the incidence seen in placebo patients, are shown in Table 1.
| Study Phase | Titration | Maintenance | Overall | |||
| EXELON
≥ 6 to 12 mg/day | Placebo | EXELON
≥ 6 to 12 mg/day | Placebo | EXELON
≥ 6 to 12 mg/day | Placebo | |
| (n = 1,189) | (n = 868) | (n = 987) | (n = 788) | (n = 1,189) | (n = 868) | |
| Event/%
Discontinuing | ||||||
| Nausea | 8 | < 1 | 1 | < 1 | 8 | 1 |
| Vomiting | 4 | < 1 | 1 | < 1 | 5 | < 1 |
| Anorexia | 2 | 0 | 1 | < 1 | 3 | < 1 |
| Dizziness | 2 | < 1 | 1 | < 1 | 2 | < 1 |
Adverse Reactions Observed at an Incidence of at Least 2%
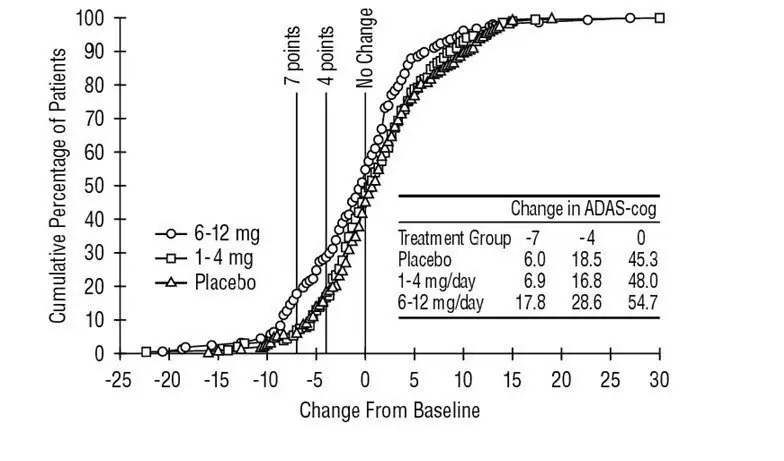
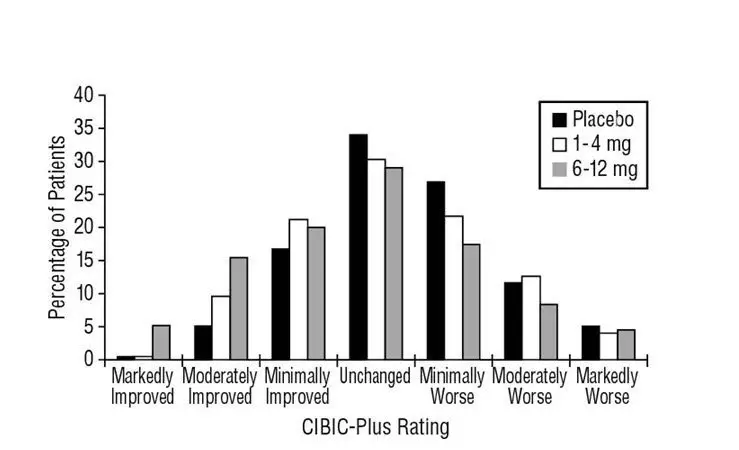
Table 2 lists adverse reactions that occurred in at least 2% of patients in placebo-controlled trials, and for which the rate of occurrence was greater for patients treated with EXELON doses of 6 mg to 12 mg per day than for those treated with placebo.
In general, adverse reactions were less frequent later in the course of treatment.
No systematic effect of race or age could be determined from the incidence of adverse reactions in the controlled studies. Nausea, vomiting and weight loss were more frequent in women than men.
|
Body System/Adverse Reaction | EXELON
(6-12 mg/day) (n = 1,189) | Placebo
(n = 868) |
| Percent of Patients with any Adverse Event | 92 | 79 |
| Autonomic Nervous System | ||
| Increased Sweating | 4 | 1 |
| Syncope | 3 | 2 |
| Body as a Whole | ||
| Fatigue | 9 | 5 |
| Asthenia | 6 | 2 |
| Malaise | 5 | 2 |
| Decreased Weight** | 3 | < 1 |
| Cardiovascular Disorders, General | ||
| Hypertension | 3 | 2 |
| Central and Peripheral Nervous System | ||
| Dizziness | 21 | 11 |
| Headache | 17 | 12 |
| Somnolence | 5 | 3 |
| Tremor | 4 | 1 |
| Gastrointestinal System | ||
| Nausea* | 47 | 12 |
| Vomiting* | 31 | 6 |
| Diarrhea | 19 | 11 |
| Anorexia*** | 17 | 3 |
| Abdominal Pain | 13 | 6 |
| Dyspepsia | 9 | 4 |
| Psychiatric Disorders | ||
| Insomnia | 9 | 7 |
| Confusion | 8 | 7 |
| Depression | 6 | 4 |
| Anxiety | 5 | 3 |
| Hallucination | 4 | 3 |
| Aggressive Reaction | 3 | 2 |
| Resistance Mechanism Disorders | ||
| Urinary Tract Infection | 7 | 6 |
*Nausea and Vomiting: In the controlled clinical trials, 47% of the patients treated with an EXELON dose in the therapeutic range of 6 mg to 12 mg per day (n = 1189) developed nausea (compared with 12% in placebo). A total of 31% of EXELON-treated patients developed at least 1 episode of vomiting (compared with 6% for placebo). The rate of vomiting was higher during the titration phase (24% versus 3% for placebo) than in the maintenance phase (14% versus 3% for placebo). The rates were higher in women than men. Five percent of patients discontinued for vomiting, compared to less than 1% for patients on placebo. Vomiting was severe in 2% of EXELON-treated patients and was rated as mild or moderate each in 14% of patients. The rate of nausea was higher during the titration phase (43% versus 9% for placebo) than in the maintenance phase (17% versus 4% for placebo).
**Weight Decreased: In the controlled trials, approximately 26% of women on high doses of EXELON (greater than 9 mg per day) had weight loss equal to or greater than 7% of their baseline weight compared to 6% in the placebo-treated patients. About 18% of the males in the high-dose group experienced a similar degree of weight loss compared to 4% in placebo-treated patients. It is not clear how much of the weight loss was associated with anorexia, nausea, vomiting, and the diarrhea associated with the drug.
***Anorexia: In the controlled clinical trials, of the patients treated with an EXELON dose of 6 mg to 12 mg per day, 17% developed anorexia compared to 3% of the placebo patients. Neither the time course nor the severity of the anorexia is known.
Mild-to-Moderate Parkinson’s Disease Dementia
EXELON has been administered to 779 individuals during clinical trials worldwide. Of these, 663 patients have been treated for at least 3 months, 476 patients have been treated for at least 6 months, and 313 patients have been treated for 1 year.
Most Common Adverse Reactions
The most common adverse reactions, defined as those occurring at a frequency of at least 5% and twice the placebo rate, are largely predicted by EXELON's cholinergic effects. These include nausea, vomiting, tremor, anorexia, and dizziness.
Discontinuation Rates
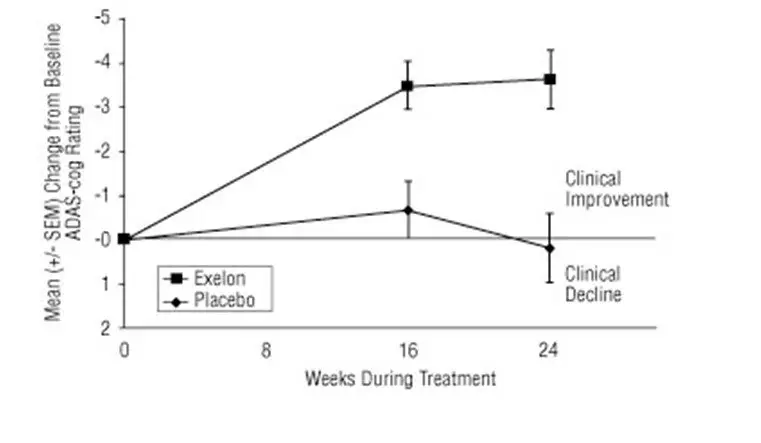
The rate of discontinuation due to adverse events in the single placebo-controlled trial of EXELON was 18% for patients receiving 3 mg to 12 mg per day compared to 11% for patients on placebo during the 24-week study.
The most frequent adverse reactions that led to discontinuation from this study, defined as those occurring in at least 1% of patients receiving EXELON and more frequent than those receiving placebo, were nausea (3.6% EXELON versus 0.6% placebo), vomiting (1.9% EXELON versus 0.6% placebo), and tremor (1.7% EXELON versus 0.0% placebo).
Adverse Reactions Observed at an Incidence of at Least 2%
Table 3 lists adverse reactions that occurred in at least 2% of patients in a single placebo-controlled trial and during the first 24 weeks of a 76-week open-label active-controlled trial for which the rate of occurrence was greater for patients treated with EXELON doses of 3 mg to 12 mg per day than for those treated with placebo in the placebo-controlled trial.
In general, adverse reactions were less frequent later in the course of treatment.
| Active-Controlled Study | Placebo-Controlled Study |
||
|
Body System/Adverse Reaction | EXELON
(3 to 12 mg/day) (n = 294) | EXELON
(3 to 12 mg/day) (n = 362) | Placebo
(n = 179) |
| Percent of Patients with any Adverse Event | 88 | 84 | 71 |
| Gastrointestinal Disorders | |||
| Nausea | 38 | 29 | 11 |
| Vomiting | 13 | 17 | 2 |
| Diarrhea | 8 | 7 | 4 |
| Upper Abdominal Pain | 4 | 4 | 1 |
| Salivary hypersecretion | 2 | 1 | 0 |
| General Disorders and Administrative Site Conditions | |||
| Fall | 10 | 6 | 6 |
| Fatigue | 5 | 4 | 3 |
| Asthenia | 4 | 2 | 1 |
| Metabolism and Nutritional Disorders | |||
| Anorexia | - | 6 | 3 |
| Decreased Appetite | 5 | 8 | 5 |
| Dehydration | 1 | 2 | 1 |
| Nervous System Disorders | |||
| Tremor | 23 | 10 | 4 |
| Dizziness | 8 | 6 | 1 |
| Headache | 4 | 4 | 3 |
| Somnolence | 6 | 4 | 3 |
| Parkinson’s Disease (worsening) | -* | 3 | 1 |
| Bradykinesia | 3 | 3 | 2 |
| Dyskinesia | 3 | 1 | 1 |
| Cogwheel rigidity | 3 | 1 | 0 |
| Hypokinesia | 2 | 1 | 0 |
| Parkinsonism | - | 2 | 1 |
| Psychiatric Disorders | |||
| Anxiety | 4 | 4 | 1 |
| Insomnia | 2 | 3 | 2 |
| Restlessness | 1 | 3 | 2 |
| Skin and Subcutaneous Tissue Disorders | |||
| Increased Sweating | 2 | 2 | 1 |
*Parkinson’s disease (worsening) in the active-controlled study was assessed by reported pre-identified adverse events (tremor, cogwheel rigidity, fall), each of them listed with corresponding frequencies.
7. Drug Interactions
7.1 Metoclopramide
Due to the risk of additive extrapyramidal adverse reactions, the concomitant use of metoclopramide and EXELON is not recommended.
7.2 Cholinomimetic and Anticholinergic Medications
EXELON may increase the cholinergic effects of other cholinomimetic medications and may also interfere with the activity of anticholinergic medications (e.g., oxybutynin, tolterodine). Concomitant use of EXELON with medications having these pharmacologic effects is not recommended unless deemed clinically necessary [see Warnings and Precautions (5.3)].
8. Use In Specific Populations
8.2 Lactation
Risk Summary
There are no data on the presence of rivastigmine in human milk, the effects on the breastfed infant, or the effects of rivastigmine on milk production. Rivastigmine and its metabolites are excreted in rat milk following oral administration of rivastigmine; levels of rivastigmine plus metabolites in rat milk are approximately 2 times that in maternal plasma.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for EXELON and any potential adverse effects on the breastfed infant from EXELON or from the underlying maternal condition.
| EXELON
rivastigmine tartrate capsule |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| EXELON
rivastigmine tartrate capsule |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| EXELON
rivastigmine tartrate capsule |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| EXELON
rivastigmine tartrate capsule |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |