Drug Detail:Exkivity (Mobocertinib)
Drug Class: EGFR inhibitors
Highlights of Prescribing Information
EXKIVITY® (mobocertinib) capsules, for oral use
Initial U.S. Approval: 2021
WARNING: QTc PROLONGATION AND TORSADES DE POINTES
See full prescribing information for complete boxed warning.
- EXKIVITY can cause life-threatening heart rate-corrected QT (QTc) prolongation, including Torsades de Pointes, which can be fatal, and requires monitoring of QTc and electrolytes at baseline and periodically during treatment. Increase monitoring frequency in patients with risk factors for QTc prolongation (5.1).
- Avoid use of concomitant drugs which are known to prolong the QTc interval and use of strong or moderate CYP3A inhibitors with EXKIVITY, which may further prolong the QTc (5.1, 7.1, 7.3).
- Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity of QTc prolongation (2.3).
Recent Major Changes
| Dosage and Administration (2.3) | 03/2023 |
Indications and Usage for Exkivity
EXKIVITY is a kinase inhibitor indicated for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy.
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). (1, 2.1)
Exkivity Dosage and Administration
Recommended Dosage: 160 mg orally once daily, with or without food. (2.2)
Dosage Forms and Strengths
Capsules: 40 mg. (3)
Contraindications
None. (4)
Warnings and Precautions
- Interstitial Lung Disease (ILD)/Pneumonitis: Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis. Immediately withhold EXKIVITY in patients with suspected ILD/pneumonitis and permanently discontinue EXKIVITY if ILD/pneumonitis is confirmed. (2.3, 5.2)
- Cardiac Toxicity: Monitor cardiac function, including left ventricular ejection fraction, at baseline and during treatment. Withhold, resume at reduced dose or permanently discontinue based on severity. (2.3, 5.3)
- Diarrhea: Diarrhea may lead to dehydration or electrolyte imbalance, with or without renal impairment. Monitor electrolytes and advise patients to start an antidiarrheal agent at first episode of diarrhea and to increase fluid and electrolyte intake. Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity. (2.3, 5.4)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective non-hormonal contraception. (5.5, 8.1, 8.3)
Adverse Reactions/Side Effects
The most common (>20%) adverse reactions are diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain. The most common (≥2%) Grade 3 or 4 laboratory abnormalities were decreased lymphocytes, increased amylase, increased lipase, decreased potassium, decreased hemoglobin, increased creatinine, and decreased magnesium. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals America, Inc. at 1-844-217-6468 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A Inhibitors: Avoid concomitant use of EXKIVITY with strong or moderate CYP3A inhibitors. If concomitant use of a moderate CYP3A inhibitor is unavoidable, reduce the dose of EXKIVITY. (2.4, 7.1)
- CYP3A Inducers: Avoid concomitant use of EXKIVITY with strong or moderate CYP3A inducers. (7.1)
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
Full Prescribing Information
WARNING: QTc PROLONGATION AND TORSADES DE POINTES
- EXKIVITY can cause life threatening heart rate-corrected QT (QTc) prolongation, including Torsades de Pointes, which can be fatal, and requires monitoring of QTc and electrolytes at baseline and periodically during treatment. Increase monitoring frequency in patients with risk factors for QTc prolongation [see Warnings and Precautions (5.1)].
- Avoid use of concomitant drugs which are known to prolong the QTc interval and use of strong or moderate CYP3A inhibitors with EXKIVITY, which may further prolong the QTc [see Warnings and Precautions (5.1), Drug Interactions (7.1, 7.3)].
- Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity of QTc prolongation [see Dosage and Administration (2.3)].
1. Indications and Usage for Exkivity
EXKIVITY is indicated for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test [see Dosage and Administration (2.1)], whose disease has progressed on or after platinum-based chemotherapy.
This indication is approved under accelerated approval based on overall response rate and duration of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
2. Exkivity Dosage and Administration
2.1 Patient Selection
Select patients with locally advanced or metastatic NSCLC for treatment with EXKIVITY based on the presence of EGFR exon 20 insertion mutations [see Clinical Studies (14)]. Information on FDA-approved tests is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
The recommended dosage of EXKIVITY is 160 mg orally once daily until disease progression or unacceptable toxicity.
Take EXKIVITY with or without food [see Clinical Pharmacology 12.3], at the same time each day. Swallow EXKIVITY capsules whole. Do not open, chew or dissolve the contents of the capsules.
If a dose is missed by more than 6 hours, skip the dose and take the next dose the following day at its regularly scheduled time.
If a dose is vomited, do not take an additional dose. Take the next dose as prescribed the following day.
2.3 Dosage Modifications for Adverse Reactions
EXKIVITY dose reduction levels for adverse reactions are summarized in Table 1.
| Dose Reductions | Dose Level |
|---|---|
| First dose reduction | 120 mg once daily |
| Second dose reduction | 80 mg once daily |
Recommended dosage modifications of EXKIVITY for adverse reactions are provided in Table 2.
| Adverse Reaction | Severity* | EXKIVITY Dosage Modification |
|---|---|---|
| ULN = upper limit of normal | ||
|
||
| QTc Interval Prolongation and Torsades de Pointes [see Warnings and Precautions (5.1)] | Grade 2 (QTc interval 481-500 msec) | First Occurrence
|
| Grade 3 (QTc interval ≥501 msec or QTc interval increase of >60 msec from baseline) | First Occurrence
|
|
| Grade 4 (Torsades de Pointes; polymorphic ventricular tachycardia; signs/symptoms of serious arrhythmia) |
|
|
| Interstitial Lung Disease (ILD)/pneumonitis [see Warnings and Precautions (5.2)] | Any grade |
|
| Decreased Ejection Fraction or Heart Failure [see Warnings and Precautions (5.3)] | Grade 2 decreased ejection fraction |
|
| ≥ Grade 2 heart failure or Grade 3 or 4 decreased ejection fraction |
|
|
| Diarrhea [see Warnings and Precautions (5.4)] | Intolerable or recurrent Grade 2 or Grade 3 |
|
| Grade 4 | First Occurrence
|
|
| Increased Amylase or Lipase [see Adverse Reactions (6.1)] | Grade 3 without signs or symptoms |
|
| Grade 3 with signs or symptoms |
|
|
| Grade 4 | First Occurrence
|
|
| Other Adverse Reactions [see Adverse Reactions (6.1)] | Intolerable or recurrent Grade 2 or Grade 3 |
|
| Grade 4 | First Occurrence
|
|
2.4 Dosage Modifications for Moderate CYP3A Inhibitors
Avoid concomitant use of moderate CYP3A inhibitors with EXKIVITY. If concomitant use of a moderate CYP3A inhibitor cannot be avoided, reduce the EXKIVITY dose by approximately 50% (i.e., from 160 to 80 mg, 120 to 40 mg, or 80 to 40 mg) and monitor the QTc interval more frequently. After the moderate CYP3A inhibitor has been discontinued for 3 to 5 elimination half-lives, resume EXKIVITY at the dose taken prior to initiating the moderate CYP3A inhibitor [see Drug Interactions (7.1)].
3. Dosage Forms and Strengths
Capsules: 40 mg, white, size 2, imprinted with "MB788" on the cap and "40mg" on the body in black ink.
5. Warnings and Precautions
5.1 QTc Prolongation and Torsades de Pointes
EXKIVITY can cause life-threatening heart rate-corrected QT (QTc) prolongation, including Torsades de Pointes, which can be fatal. In the 250 patient subset of the pooled EXKIVITY safety population who had scheduled and unscheduled electrocardiograms (ECGs) [see Adverse Reactions (6.1), Clinical Pharmacology (12.2)], 1.2% of patients had a QTc interval >500 msec and 11% of patients had a change-from-baseline QTc interval >60 msec. Grade 4 Torsades de Pointes occurred in 1 patient (0.4%). Clinical trials of EXKIVITY did not enroll patients with baseline QTc greater than 470 msec.
Assess QTc and electrolytes at baseline and correct abnormalities in sodium, potassium, calcium, and magnesium prior to initiating EXKIVITY. Monitor QTc and electrolytes periodically during treatment. Increase monitoring frequency in patients with risk factors for QTc prolongation, such as patients with congenital long QT syndrome, heart disease, or electrolyte abnormalities. Avoid use of concomitant drugs which are known to prolong the QTc interval. Avoid concomitant use of strong or moderate CYP3A inhibitors with EXKIVITY [see Drug Interactions (7.1)], which may further prolong the QTc [see Drug Interactions (7.3)].
Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity of the QTc prolongation [see Dosage and Administration (2.3)].
5.2 Interstitial Lung Disease (ILD)/Pneumonitis
EXKIVITY can cause ILD/pneumonitis, which can be fatal. In the pooled EXKIVITY safety population [see Adverse Reactions (6.1)], ILD/pneumonitis occurred in 4.3% of patients including 0.8% Grade 3 events and 1.2% fatal events.
Monitor patients for new or worsening pulmonary symptoms indicative of ILD/pneumonitis. Immediately withhold EXKIVITY in patients with suspected ILD/pneumonitis and permanently discontinue EXKIVITY if ILD/pneumonitis is confirmed [see Dosage and Administration (2.3)].
5.3 Cardiac Toxicity
EXKIVITY can cause cardiac toxicity (including decreased ejection fraction, cardiomyopathy, and congestive heart failure) resulting in heart failure which can be fatal. In the pooled EXKIVITY safety population [see Adverse Reactions (6.1)], heart failure occurred in 2.7% of patients including 1.2% Grade 3 reactions, 0.4% Grade 4 reactions, and one (0.4%) fatal case of heart failure.
EXKIVITY can cause QTc prolongation resulting in Torsades de Pointes [see Warnings and Precautions (5.1)]. Atrial fibrillation (1.6%), ventricular tachycardia (0.4%), first degree atrioventricular block (0.4%), second degree atrioventricular block (0.4%), left bundle branch block (0.4%), supraventricular extrasystoles (0.4%) and ventricular extrasystoles (0.4%) also occurred in patients receiving EXKIVITY.
Monitor cardiac function, including assessment of left ventricular ejection fraction at baseline and during treatment. Withhold, reduce the dose, or permanently discontinue EXKIVITY based on the severity [see Dosage and Administration (2.3)].
5.4 Diarrhea
EXKIVITY can cause diarrhea, which can be severe. In the pooled EXKIVITY safety population [see Adverse Reactions (6.1)], diarrhea occurred in 93% of patients, including 20% Grade 3 and 0.4% Grade 4. The median time to first onset of diarrhea was 5 days but diarrhea has occurred within 24 hours after administration of EXKIVITY. In the 48% of patients whose diarrhea resolved, the median time to resolution was 3 days. Diarrhea may lead to dehydration or electrolyte imbalance, with or without renal impairment. Treat diarrhea promptly.
Advise patients to start an antidiarrheal agent (e.g., loperamide) at first sign of diarrhea or increased bowel movement frequency and to increase fluid and electrolyte intake.
Monitor electrolytes and withhold, reduce the dose or permanently discontinue EXKIVITY based on the severity [see Dosage and Administration (2.3)].
5.5 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, EXKIVITY can cause fetal harm when administered to a pregnant woman. Oral administration of mobocertinib to pregnant rats during the period of organogenesis resulted in embryolethality at maternal exposures approximately 1.7 times the human exposure based on area under the curve (AUC) at the 160 mg once daily clinical dose.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective non-hormonal contraception during treatment with EXKIVITY [see Drug Interactions (7.2)] and for 1 month after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with EXKIVITY and for 1 week after the last dose of EXKIVITY [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- QTc Prolongation and Torsades de Pointes [see Warnings and Precautions (5.1)]
- Interstitial Lung Disease (ILD)/Pneumonitis [see Warnings and Precautions (5.2)]
- Cardiac Toxicity [see Warnings and Precautions (5.3)]
- Diarrhea [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in WARNINGS AND PRECAUTIONS reflects exposure to EXKIVITY as a single agent at a dose of 160 mg orally once daily in 256 patients, including 114 patients with EGFR exon 20 insertion mutation-positive locally advanced or metastatic NSCLC from Study AP32788-15-101, and patients with other solid tumors. Forty-eight percent (48%) were exposed for 6 months or longer and 12% were exposed for greater than one year. The most common (>20%) adverse reactions were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain. The most common (≥2%) Grade 3 or 4 laboratory abnormalities were decreased lymphocytes, increased amylase, increased lipase, decreased potassium, decreased hemoglobin, increased creatinine, and decreased magnesium.
7. Drug Interactions
7.1 Effect of Other Drugs on EXKIVITY
| Strong or Moderate CYP3A Inhibitors | |
| Clinical Impact |
|
| Prevention or Management |
|
| Strong or Moderate CYP3A Inducers | |
| Clinical Impact |
|
| Prevention or Management |
|
7.2 Effect of EXKIVITY on Other Drugs
| CYP3A Substrates | |
| Clinical Impact |
|
| Prevention or Management |
|
7.3 Drugs that Prolong the QTc Interval
| Drugs that Prolong the QTc Interval | |
|---|---|
| Clinical Impact |
|
| Prevention or Management |
|
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
EXKIVITY can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of EXKIVITY in pediatric patients have not been established.
8.5 Geriatric Use
Of the 114 patients [see Clinical Studies (14)] who received EXKIVITY in clinical studies, 37% were 65 years and over, and 7% were 75 years and over. No overall difference in effectiveness was observed between patients aged 65 and older and younger patients. Exploratory analysis suggests a higher incidence of Grade 3 and 4 adverse reactions (69% vs 47%) and serious adverse reactions (64% vs 35%) in patients 65 years and older as compared to those younger than 65 years.
8.6 Renal Impairment
No dosage adjustment of EXKIVITY is recommended for patients with mild to moderate renal impairment (estimated glomerular filtration rate [eGFR] 30 to 89 mL/min/1.73 m2 by Modification of Diet in Renal Disease [MDRD] equation). The recommended dosage of EXKIVITY has not been established for patients with severe renal impairment (eGFR <30 mL/min/1.73 m2) [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment of EXKIVITY is recommended for patients with mild (total bilirubin ≤ upper limit of normal [ULN] and aspartate aminotransferase [AST] > ULN or total bilirubin >1 to 1.5 times ULN and any AST) or moderate hepatic impairment (total bilirubin ≥1.5 to 3 times ULN and any AST). The recommended dosage of EXKIVITY has not been established for patients with severe hepatic impairment (total bilirubin >3 times ULN and any AST) [see Clinical Pharmacology (12.3)].
11. Exkivity Description
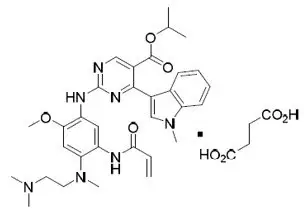
Mobocertinib is a kinase inhibitor. The chemical name for mobocertinib succinate is propan-2-yl 2-[5-(acryloylamino)-4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxyanilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate succinate. The molecular formula is C32H39N7O4 + C4H6O4 (succinate salt) which corresponds to a molecular weight of 703.8 g/mol. Mobocertinib has no chiral centers. The chemical structure of mobocertinib succinate is shown below:
Mobocertinib succinate has a solubility of 152 mg/mL in pH 1.0 and >17.6 mg/mL in pH 6.8 solutions at 37°C.
EXKIVITY capsule for oral administration contains 40 mg mobocertinib equivalent to 48.06 mg mobocertinib succinate, with no inactive ingredients. The capsule shells contain gelatin and titanium dioxide. The printing ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water.
12. Exkivity - Clinical Pharmacology
12.1 Mechanism of Action
Mobocertinib is a kinase inhibitor of the epidermal growth factor receptor (EGFR) that irreversibly binds to and inhibits EGFR exon 20 insertion mutations at lower concentrations than wild type (WT) EGFR. Two pharmacologically-active metabolites (AP32960 and AP32914) with similar inhibitory profiles to mobocertinib have been identified in the plasma after oral administration of mobocertinib. In vitro, mobocertinib also inhibited the activity of other EGFR family members (HER2 and HER4) and one additional kinase (BLK) at clinically relevant concentrations (IC50 values <2 nM).
In cultured cell models, mobocertinib inhibited the proliferation of cells driven by different EGFR exon 20 insertion mutation variants at 1.5- to 10-fold lower concentrations than WT-EGFR signaling inhibition.
In animal tumor implantation models, mobocertinib exhibited anti-tumor activity against xenografts with the EGFR exon 20 insertions NPH or ASV.
12.2 Pharmacodynamics
Mobocertinib exposure-response relationships and the time course of pharmacodynamic response are unknown.
12.3 Pharmacokinetics
After single- and multiple-dose administration, combined molar Cmax and AUC0-24h of mobocertinib and its active metabolites, AP32960 and AP32914, was dose-proportional over the dose range of 5 to 180 mg once daily (0.03 to 1.1 times the approved recommended dosage). No clinically meaningful accumulation was observed after administration of EXKIVITY 160 mg once daily based on the AUC ratio of mobocertinib.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were not performed with mobocertinib. Mobocertinib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay and did not induce chromosomal aberrations in an in vitro chromosome aberration assay in human peripheral blood lymphocytes. Mobocertinib was not clastogenic in an in vivo bone marrow micronucleus test in rats.
Fertility, early embryonic development, and pre- and post-natal toxicology studies were not conducted with mobocertinib; however, in 4- and 13-week repeat-dose toxicology studies in rats and dogs, there were generally reversible changes that included decreases in organ weights affecting multiple reproductive organs (including ovaries, seminal vesicle/prostate gland, and/or uterus) at exposures ≥0.3 times the AUC observed at the recommended clinical dose of 160 mg once daily, as well as microscopic changes of decreased epithelial thickness/inflammation of the cervix/vagina and atrophy of the uterus, prostate gland, or mammary gland (males only) at exposures ≥0.2 times the AUC at the 160 mg once daily clinical dose in rats and/or dogs. Based on these findings, mobocertinib may impair fertility in males and females of reproductive potential. These effects may be reversible.
13.2 Animal Toxicology and/or Pharmacology
In rats, mobocertinib administration resulted in histological findings of decreased corneal epithelial thickness in the 4- and 13-week repeat-dose toxicology studies at doses ≥0.8 times the human exposure (AUC) at the 160 mg once daily clinical dose. In the 4-week repeat-dose study in dogs, mobocertinib administration resulted in discharge from the eye, sclera injection, partial or complete closure of the eye and histological findings of corneal epithelial atrophy at doses ≥0.3 times the AUC at the 160 mg once daily clinical dose. In the 13-week repeat-dose study in dogs, mobocertinib administration resulted in discharge, conjunctival hyperemia, and corneal opacity correlating histologically with decreased corneal epithelial thickness at doses ≥0.2 times the AUC at the 160 mg once daily clinical dose. The clinical relevance of these findings is unknown.
14. Clinical Studies
The efficacy of EXKIVITY was evaluated in a pooled subset of patients with EGFR exon 20 insertion mutation-positive metastatic or locally advanced NSCLC whose disease had progressed on or after platinum-based chemotherapy enrolled in an international, open-label, multicohort clinical trial (AP32788-15-101, NCT02716116). Patients had histologically or cytologically confirmed locally advanced or metastatic disease (Stage IIIB or IV) and a documented EGFR exon 20 insertion mutation based on local testing. Patients received EXKIVITY at a dose of 160 mg once daily until disease progression or intolerable toxicity.
In the efficacy population, EGFR exon 20 insertion mutation status was determined by prospective local testing using samples from tumor tissue (87%), plasma (5%), or other specimens such as pleural fluid (8%). Of the 114 patients with EGFR exon 20 insertion mutations, 70% of patient tissue samples were tested retrospectively using Life Technologies Corporation Oncomine Dx™ Target Test. While 75% of patients were positive for EGFR exon 20 insertion mutation, 14% did not have an EGFR exon 20 insertion mutation identified, and 11% did not generate reportable results.
The efficacy population consisted of 114 patients and had the following demographic characteristics: the median age was 60 years (range: 27 to 84 years); 66% were female; 60% were Asian, 37% were White, and 3% were Black; 71% had never smoked; at baseline, 75% had Eastern Cooperative Oncology Group (ECOG) performance status 1. At baseline, 99% of patients had metastatic disease, 98% of patients had adenocarcinoma histology and 35% of patients had brain metastases. The median number of prior therapies was 2 (range: 1 to 7) and 43% percent had received prior immunotherapy.
The major efficacy outcome measure was overall response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1) as evaluated by blinded independent central review (BICR). Additional efficacy outcome measures included duration of response (DOR) by BICR.
Efficacy results are summarized in Table 5.
| EXKIVITY (n=114) |
|
|---|---|
|
|
| Overall Response Rate (ORR)* (95% CI) | 28% (20, 37)† |
| Duration of Response (DOR) | |
| Median (months)‡, (95% CI) | 17.5 (7.4, 20.3) |
| Patients with DOR ≥6 months§ | 59% |
Investigator-assessed ORR was 35% (95% CI: 26, 45) with a median DOR of 11.2 months (63% of these patients had observed responses lasting longer than 6 months).
16. How is Exkivity supplied
EXKIVITY is supplied as 40 mg capsules: white, size 2, imprinted with "MB788" on the cap and "40mg" on the body in black ink.
| Bottle of 90 capsules | NDC 63020-040-90 |
| Bottle of 120 capsules | NDC 63020-040-12 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| EXKIVITY
mobocertinib capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc. | 053217022 | PACK(63020-040) , LABEL(63020-040) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Takeda Ireland Limited | 988980314 | PACK(63020-040) , LABEL(63020-040) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lonza Tampa LLC | 628112240 | MANUFACTURE(63020-040) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Lancaster Laboratories, Inc. | 069777290 | ANALYSIS(63020-040) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ash Stevens LLC | 049265333 | API MANUFACTURE(63020-040) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Micron Technologies, Inc. | 015966157 | PARTICLE SIZE REDUCTION(63020-040) | |





