Drug Detail:Extavia (Interferon beta-1b [ in-ter-fear-on-bay-ta-1b ])
Drug Class: Interferons
Highlights of Prescribing Information
EXTAVIA® (interferon beta-1b) for injection, for subcutaneous use
Initial U.S. Approval: 1993
Recent Major Changes
|
Warnings and Precautions, Pulmonary Arterial Hypertension (5.8) |
7/2023 |
Indications and Usage for Extavia
EXTAVIA is an interferon beta indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. (1)
Extavia Dosage and Administration
- For subcutaneous use only. (2.1)
- The recommended dose is 0.25 mg every other day. Generally, start at 0.0625 mg (0.25 mL) every other day, and increase over a six-week period to 0.25 mg (1 mL) every other day. (2.1)
- Reconstitute lyophilized powder with supplied diluent; the removable diluent cap contains natural rubber latex. (2.2)
Dosage Forms and Strengths
For injection: 0.3 mg of lyophilized powder in a single-dose vial for reconstitution. (3)
Contraindications
History of hypersensitivity to natural or recombinant interferon beta, albumin (human) or mannitol. (4)
Warnings and Precautions
- Hepatic Injury: Monitor liver function tests and signs and symptoms of hepatic injury; consider discontinuing EXTAVIA if serious hepatic injury occurs. (5.1, 5.11)
- Anaphylaxis and Other Allergic Reactions: Discontinue if anaphylaxis occurs. (5.2)
- Depression and Suicide: Advise patients to immediately report any symptom of depression and/or suicidal ideation; consider discontinuation of EXTAVIA if depression occurs. (5.3)
- Congestive Heart Failure (CHF): Monitor patients with CHF for worsening of cardiac symptoms; consider discontinuation of EXTAVIA if worsening of CHF occurs. (5.4)
- Injection Site Reactions Including Necrosis: Do not administer EXTAVIA into affected area until fully healed; if multiple lesions occur, change injection site or discontinue EXTAVIA until healing of skin lesions. (5.5)
- Leukopenia: Monitor complete blood count. (5.6, 5.12)
- Thrombotic Microangiopathy (TMA): Cases of TMA have been reported. Discontinue EXTAVIA if clinical symptoms and laboratory findings consistent with TMA occur and a relationship to EXTAVIA is suspected. (5.7)
- Pulmonary Arterial Hypertension: Cases of pulmonary arterial hypertension (PAH) have been reported in patients treated with interferon beta products, including EXTAVIA. Discontinue EXTAVIA if PAH is diagnosed. (5.8)
- Flu-like Symptom Complex: Consider analgesics and/or antipyretics on injection days. (5.9)
- Drug-induced Lupus Erythematosus: Cases of drug-induced lupus erythematosus have been reported. Discontinue EXTAVIA if patients develop new characteristic signs and symptoms. (5.11)
Adverse Reactions/Side Effects
In controlled clinical trials, the most common adverse reactions (at least 5% more frequent on interferon beta-1b than on placebo) were: injection site reaction, lymphopenia, flu-like symptoms, myalgia, leukopenia, neutropenia, increased liver enzymes, headache, hypertonia, pain, rash, insomnia, abdominal pain, and asthenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Pregnancy: Based on animal data, may cause fetal harm. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2023
Related/similar drugs
Copaxone, Aubagio, Gilenya, Tecfidera, Avonex, TysabriFull Prescribing Information
1. Indications and Usage for Extavia
EXTAVIA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
2. Extavia Dosage and Administration
2.1 Dosing Information
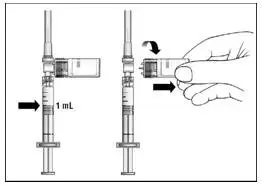
The recommended starting dose is 0.0625 mg (0.25 mL) subcutaneously every other day, with dose increases over a six-week period to the recommended dose of 0.25 mg (1 mL) every other day (see Table 1).
| aDosed every other day, subcutaneously. | |||
|
EXTAVIA dosea |
Percentage of |
Volume |
|
|
Weeks 1-2 |
0.0625 mg |
25% |
0.25 mL |
|
Weeks 3-4 |
0.125 mg |
50% |
0.5 mL |
|
Weeks 5-6 |
0.1875 mg |
75% |
0.75 mL |
|
Week 7 and thereafter |
0.25 mg |
100% |
1 mL |
If a dose of EXTAVIA is missed, then it should be taken as soon as the patient remembers or is able to take it. The patient should not take EXTAVIA on two consecutive days. The next injection should be taken about 48 hours (two days) after that dose. If the patient accidentally takes more than their prescribed dose, or takes it on two consecutive days, they should be instructed to call their healthcare provider immediately.
2.2 Reconstitution of the Lyophilized Powder
a) Prior to reconstitution, verify that the vial containing lyophilized EXTAVIA is not cracked or damaged. Do not use cracked or damaged vials.
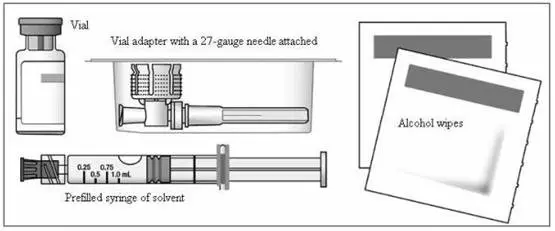
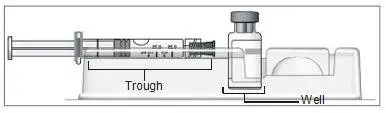
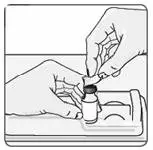
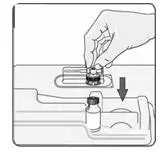
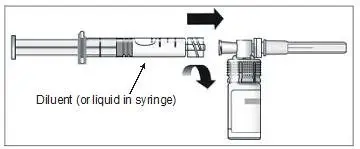
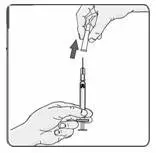
b) To reconstitute lyophilized EXTAVIA for injection, attach the pre-filled syringe containing the diluent (0.54% sodium chloride solution, USP) to the EXTAVIA vial using the vial adapter.
The removable rubber cap of the diluent (0.54% sodium chloride solution, USP) pre-filled syringe contains natural rubber latex, which may cause allergic reactions and should not be handled by latex-sensitive individuals.
c) Slowly inject 1.2 mL of diluent into the EXTAVIA vial.
d) Gently swirl the vial to dissolve the lyophilized powder completely; do not shake. Foaming may occur during reconstitution or if the vial is swirled or shaken too vigorously. If foaming occurs, allow the vial to sit undisturbed until the foam settles.
e) 1 mL of reconstituted EXTAVIA solution contains 0.25 mg of interferon beta-1b.
f) After reconstitution, if not used immediately, refrigerate the reconstituted EXTAVIA solution at 35°F to 46°F (2°C to 8°C) and use within three hours. Do not freeze.
2.3 Important Administration Instructions
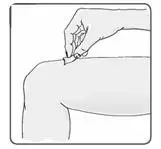
a) Perform the first EXTAVIA injection under the supervision of an appropriately qualified healthcare professional. If patients or caregivers are to administer EXTAVIA, train them in the proper subcutaneous injection technique and assess their ability to inject subcutaneously to ensure the proper administration of EXTAVIA.
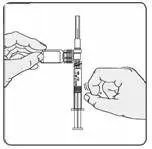
b) Visually inspect the reconstituted EXTAVIA solution before use; discard if it contains particulate matter or is discolored.
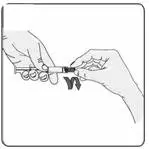
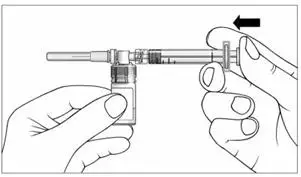
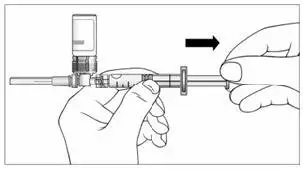
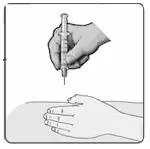
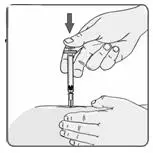
c) Keeping the syringe and vial adapter in place, turn the assembly over so that the vial is on top. Withdraw the appropriate dose of EXTAVIA solution. Remove the vial from the vial adapter before injecting EXTAVIA.
d) Use safe disposal procedures for needles and syringes.
e) Do not re-use needles or syringes.
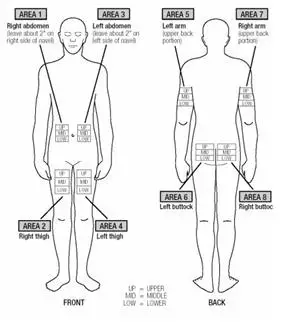
f) Advise patients and caregivers to rotate sites for subcutaneous injections to minimize the likelihood of severe injection site reactions, including necrosis or localized infection [see Warnings and Precautions (5.5)].
3. Dosage Forms and Strengths
For injection: 0.3 mg white to off-white lyophilized powder in a single-dose vial for reconstitution.
4. Contraindications
EXTAVIA is contraindicated in patients with a history of hypersensitivity to natural or recombinant interferon beta, albumin (human), or any other component of the formulation.
5. Warnings and Precautions
5.1 Hepatic Injury
Severe hepatic injury, including cases of hepatic failure, some of which have been due to autoimmune hepatitis, has been rarely reported in patients taking EXTAVIA. In some cases, these events have occurred in the presence of other drugs or comorbid medical conditions that have been associated with hepatic injury. Consider the potential risk of EXTAVIA used in combination with known hepatotoxic drugs or other products (e.g., alcohol) prior to EXTAVIA administration, or when adding new agents to the regimen of patients already on EXTAVIA. Monitor patients for signs and symptoms of hepatic injury. Consider discontinuing EXTAVIA if serum transaminase levels significantly increase, or if they are associated with clinical symptoms, such as jaundice.
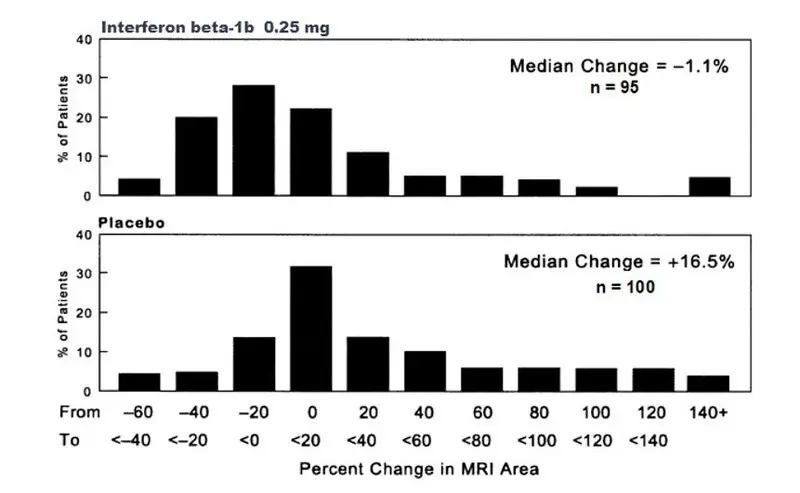
Asymptomatic elevation of serum transaminases is common in patients treated with EXTAVIA. In controlled clinical trials, elevations of serum glutamic-pyruvic transaminase (SGPT) to greater than five times baseline value were reported in 12% of patients receiving interferon beta-1b (compared to 4% on placebo), and increases of serum glutamic-oxaloacetic transaminase (SGOT) to greater than five times baseline value were reported in 4% of patients receiving interferon beta-1b (compared to 1% on placebo), leading to dose-reduction or discontinuation of treatment in some patients [see Adverse Reactions (6.1)]. Monitor liver function tests [see Warnings and Precautions (5.11)].
5.2 Anaphylaxis and Other Allergic Reactions
Anaphylaxis has been reported as a rare complication of interferon beta-1b use. Other allergic reactions have included dyspnea, bronchospasm, tongue edema, skin rash, and urticaria [see Adverse Reactions (6.1)]. Discontinue EXTAVIA if anaphylaxis occurs.
The removable rubber cap of the diluent (0.54% sodium chloride solution, USP) pre-filled syringe contains natural rubber latex, which may cause allergic reactions and should not be handled by latex-sensitive individuals. The safe use of EXTAVIA pre-filled syringe in latex-sensitive individuals has not been studied.
5.3 Depression and Suicide
Depression and suicide have been reported to occur with increased frequency in patients receiving interferon beta products, including interferon beta-1b. Advise patients to report any symptom of depression and/or suicidal ideation to their healthcare provider. If a patient develops depression, discontinuation of EXTAVIA therapy should be considered.
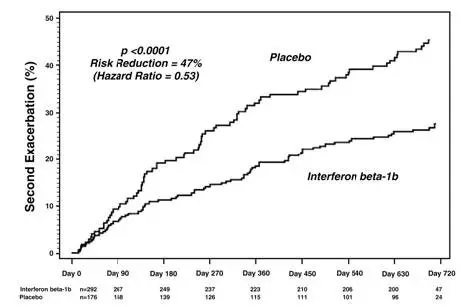
In randomized controlled clinical trials, there were three suicides and eight suicide attempts among the 1532 patients on interferon beta-1b compared to one suicide and four suicide attempts among 965 patients on placebo.
5.4 Congestive Heart Failure
Monitor patients with preexisting congestive heart failure (CHF) for worsening of their cardiac condition during initiation of and continued treatment with EXTAVIA. While beta interferons do not have any known direct-acting cardiac toxicity, cases of CHF, cardiomyopathy, and cardiomyopathy with CHF have been reported in patients without known predisposition to these events, and without other known etiologies being established. In some cases, these events have been temporally related to the administration of interferon beta-1b. Recurrence upon rechallenge was observed in some patients. Consider discontinuation of EXTAVIA if worsening of CHF occurs with no other etiology.
5.5 Injection Site Reactions Including Necrosis
Injection site reactions, including injection site necrosis, can occur with the use of interferon beta products, including EXTAVIA. Injection site necrosis (ISN) was reported in 4% of interferon beta-1b-treated patients in controlled clinical trials (compared to 0% on placebo) [see Adverse Reactions (6.1)]. Typically, ISN occurs within the first four months of therapy, although postmarketing reports have been received of ISN occurring over one year after initiation of therapy. The necrotic lesions are typically 3 cm or less in diameter, but larger areas have been reported. Generally the necrosis has extended only to subcutaneous fat, but has extended to the fascia overlying muscle. In some lesions where biopsy results are available, vasculitis has been reported. For some lesions, debridement, and/or skin grafting have been required. In most cases, healing was associated with scarring.
In controlled clinical trials, injection site reactions occurred in 78% of patients receiving interferon beta-1b with injection site necrosis in 4%. Injection site inflammation (42%), injection site pain (16%), injection site hypersensitivity (4%), injection site necrosis (4%), injection site mass (2%), injection site edema (2%), and nonspecific reactions were significantly associated with interferon beta-1b treatment. The incidence of injection site reactions tended to decrease over time. Approximately 69% of patients experienced injection site reactions during the first three months of treatment, compared to approximately 40% at the end of the studies.
Injection site abscesses and cellulitis have been reported in the postmarketing setting with use of interferon beta products including EXTAVIA. Some cases required treatment with hospitalization for surgical drainage and intravenous antibiotics. Periodically evaluate patient understanding and use of aseptic self-injection techniques and procedures, particularly if injection site necrosis has occurred. Patients should be advised of the importance of rotating injection sites with each dose. Whether to discontinue therapy following a single site of necrosis is dependent on the extent of necrosis. For patients who continue therapy with EXTAVIA after injection site necrosis has occurred, avoid administration of EXTAVIA into the affected area until it is fully healed. If multiple lesions occur, change injection site or discontinue therapy until healing occurs.
5.6 Leukopenia
In controlled clinical trials, leukopenia was reported in 18% of patients receiving interferon beta-1b (compared to 6% on placebo), leading to a reduction of the dose of interferon beta-1b in some patients [see Adverse Reactions (6.1)]. Monitoring of complete blood and differential white blood cell counts is recommended. Patients with myelosuppression may require more intensive monitoring of complete blood cell counts, with differential and platelet counts.
5.7 Thrombotic Microangiopathy
Cases of thrombotic microangiopathy (TMA), including thrombotic thrombocytopenic purpura and hemolytic uremic syndrome, some fatal, have been reported with interferon beta products, including EXTAVIA.
Cases have been reported several weeks to years after starting interferon beta products. If clinical symptoms and laboratory findings consistent with TMA occur and a relationship to EXTAVIA is suspected, discontinue treatment and manage as clinically indicated.
5.8 Pulmonary Arterial Hypertension
Cases of pulmonary arterial hypertension (PAH) have been reported with interferon beta products, including EXTAVIA. PAH has occurred in patients treated with interferon beta products in the absence of other contributory factors. Many of the reported cases required hospitalization, including one case with interferon beta in which the patient underwent a lung transplant. PAH has developed at various time points after initiating therapy with interferon beta products and may occur several years after starting treatment.
Patients who develop unexplained symptoms (e.g., dyspnea, new or increasing fatigue) should be assessed for PAH. If alternative etiologies have been ruled out and a diagnosis of PAH is confirmed, discontinue treatment and manage as clinically indicated.
5.9 Flu-like Symptom Complex
In controlled clinical trials, the rate of flu-like symptom complex for patients on interferon beta-1b was 57% [see Adverse Reactions (6.1)]. The incidence decreased over time, with 10% of patients reporting flu-like symptom complex at the end of the studies. The median duration of flu-like symptom complex in Study 1 was 7.5 days [see Clinical Studies (14)]. Analgesics and/or antipyretics on treatment days may help ameliorate flu-like symptoms associated with EXTAVIA use.
5.10 Seizures
Seizures have been temporally associated with the use of beta interferons in clinical trials and postmarketing safety surveillance. It is not known whether these events were related to a primary seizure disorder, the effects of MS alone, the use of beta interferons, other potential precipitants of seizures (e.g., fever), or to some combination of these.
5.11 Drug-induced Lupus Erythematosus
Cases of drug-induced lupus erythematosus have been reported with some interferon beta products, including EXTAVIA. Signs and symptoms of drug-induced lupus reported in EXTAVIA-treated patients have included rash, serositis, polyarthritis, nephritis, and Raynaud’s phenomenon. Cases have occurred with positive serologic testing (including positive anti-nuclear and/or anti-double-stranded DNA antibody testing). If EXTAVIA-treated patients develop new signs and symptoms characteristic of this syndrome, EXTAVIA therapy should be stopped.
5.12 Monitoring for Laboratory Abnormalities
In addition to those laboratory tests normally required for monitoring patients with MS, complete blood and differential white blood cell counts, platelet counts, and blood chemistries, including liver function tests, are recommended at regular intervals (one, three, and six months) following introduction of EXTAVIA therapy, and then periodically thereafter in the absence of clinical symptoms.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in more details in other sections of labeling:
- Hepatic Injury [see Warnings and Precautions (5.1)]
- Anaphylaxis and Other Allergic Reactions [see Warnings and Precautions (5.2)]
- Depression and Suicide [see Warnings and Precautions (5.3)]
- Congestive Heart Failure [see Warnings and Precautions (5.4)]
- Injection Site Reactions Including Necrosis [see Warnings and Precautions (5.5)]
- Leukopenia [see Warnings and Precautions (5.6)]
- Thrombotic Microangiopathy [see Warnings and Precautions (5.7)]
- Pulmonary Arterial Hypertension [see Warnings and Precautions (5.8)]
- Flu-like Symptom Complex [see Warnings and Precautions (5.9)]
- Seizures [see Warnings and Precautions (5.10)]
- Drug-induced Lupus Erythematosus [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions and over varying lengths of time, adverse reaction rates observed in the clinical trials of interferon beta-1b cannot be directly compared to rates in clinical trials of other drugs, and may not reflect the rates observed in practice.
Among 1407 patients with MS treated with interferon beta-1b 0.25 mg every other day (including 1261 patients treated for greater than one year), the most commonly reported adverse reactions (at least 5% more frequent on interferon beta-1b than on placebo) were injection site reaction, lymphopenia, flu-like symptoms, myalgia, leukopenia, neutropenia, increased liver enzymes, headache, hypertonia, pain, rash, insomnia, abdominal pain, and asthenia. The most frequently reported adverse reactions resulting in clinical intervention (for example, discontinuation of interferon beta-1b, adjustment in dosage, or the need for concomitant medication to treat an adverse reaction symptom) were depression, flu-like symptom complex, injection site reactions, leukopenia, increased liver enzymes, asthenia, hypertonia, and myasthenia.
Table 2 enumerates adverse reactions and laboratory abnormalities that occurred among patients treated with 0.25 mg of interferon beta-1b every other day by subcutaneous injection in the pooled placebo-controlled trials (Studies 1-4) at an incidence that was at least 2% more than that observed in the placebo-treated patients [see Clinical Studies (14)].
| Abbreviations: SGPT, serum glutamic-pyruvic transaminase; SGOT, serum glutamic-oxaloacetic transaminase. a"Injection site reaction" comprises all adverse reactions occurring at the injection site (except injection site necrosis), that is, the following terms: injection site reaction, injection site hemorrhage, injection site hypersensitivity, injection site inflammation, injection site mass, injection site pain, injection site edema and injection site atrophy. b"Flu-like symptom (complex)" denotes flu syndrome and/or a combination of at least two adverse reactions from fever, chills, myalgia, malaise, sweating. |
||
| Adverse Reaction | Placebo
(N = 965) | Interferon beta-1b
(N = 1407) |
| Blood and lymphatic system disorders | ||
| Lymphocytes count decreased (< 1500/mm3) | 66% | 86% |
| Absolute neutrophil count decreased (< 1500/mm3) | 5% | 13% |
| White blood cell count decreased (< 3000/mm3) | 4% | 13% |
| Lymphadenopathy | 3% | 6% |
| Nervous system disorders | ||
| Headache | 43% | 50% |
| Insomnia | 16% | 21% |
| Incoordination | 15% | 17% |
| Vascular disorders | ||
| Hypertension | 4% | 6% |
| Respiratory, thoracic, and mediastinal disorders | ||
| Dyspnea | 3% | 6% |
| Gastrointestinal disorders | ||
| Abdominal pain | 11% | 16% |
| Hepatobiliary disorders | ||
| Alanine aminotransferase increased (SGPT > 5 times baseline) | 4% | 12% |
| Aspartate aminotransferase increased (SGOT > 5 times baseline) | 1% | 4% |
| Skin and subcutaneous tissue disorders | ||
| Rash | 15% | 21% |
| Skin disorder | 8% | 10% |
| Musculoskeletal and connective tissue disorders | ||
| Hypertonia | 33% | 40% |
| Myalgia | 14% | 23% |
| Renal and urinary disorders | ||
| Urinary urgency | 8% | 11% |
| Reproductive system and breast disorders | ||
| Metrorrhagia | 7% | 9% |
| Impotence | 6% | 8% |
| General disorders and administration-site conditions | ||
| Injection site reactiona | 26% | 78% |
| Asthenia | 48% | 53% |
| Flu-like symptoms (complex)b | 37% | 57% |
| Pain | 35% | 42% |
| Fever | 19% | 31% |
| Chills | 9% | 21% |
| Peripheral edema | 10% | 12% |
| Chest pain | 6% | 9% |
| Malaise | 3% | 6% |
| Injection site necrosis | 0% | 4% |
In addition to the adverse reactions listed in Table 2, the following adverse reactions occurred more frequently on interferon beta-1b than on placebo, but with a difference smaller than 2%: alopecia, anxiety, arthralgia, constipation, diarrhea, dizziness, dyspepsia, dysmenorrhea, leg cramps, menorrhagia, myasthenia, nausea, nervousness, palpitations, peripheral vascular disorder, prostatic disorder, tachycardia, urinary frequency, vasodilatation, and weight increase.
Laboratory Abnormalities
In the four clinical trials (Studies 1, 2, 3, and 4), leukopenia was reported in 18% and 6% of patients in interferon beta-1b- and placebo-treated groups, respectively. No patients were withdrawn or dose-reduced for neutropenia in Study 1. Three percent (3%) of patients in Studies 2 and 3 experienced leukopenia and were dose-reduced. Other abnormalities included increase of SGPT to greater than five times baseline value (12%), and increase of SGOT to greater than five times baseline value (4%). In Study 1, two patients were dose-reduced for increased hepatic enzymes; one continued on treatment and one was ultimately withdrawn. In Studies 2 and 3, 1.5% of interferon beta-1b patients were dose-reduced or interrupted treatment for increased hepatic enzymes. In Study 4, 1.7% of patients were withdrawn from treatment due to increased hepatic enzymes, two of them after a dose reduction. In Studies 1 to 4, nine (0.6%) patients were withdrawn from treatment with interferon beta-1b for any laboratory abnormality, including four (0.3%) patients following dose reduction.
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. Serum samples were monitored for the development of antibodies to interferon beta-1b during Study 1. In patients receiving 0.25 mg every other day, 56/124 (45%) were found to have serum neutralizing activity at one or more of the time points tested. In Study 4, neutralizing activity was measured every 6 months and at end of study. At individual visits after start of therapy, activity was observed in 17% up to 25% of the interferon beta-1b-treated patients. Such neutralizing activity was measured at least once in 75 (30%) out of 251 interferon beta-1b patients who provided samples during treatment phase; of these, 17 (23%) converted to negative status later in the study. Based on all the available evidence, the relationship between antibody formation and clinical safety or efficacy is not known.
These data reflect the percentage of patients whose test results were considered positive for antibodies to interferon beta-1b using a biological neutralization assay that measures the ability of immune sera to inhibit the production of the interferon-inducible protein, MxA. Neutralization assays are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of neutralizing activity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to interferon beta-1b with the incidence of antibodies to other products may be misleading.
Anaphylactic reactions have been reported with the use of interferon beta-1b [see Warnings and Precautions (5.2)].
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Although there have been no well-controlled studies in pregnant women, available data, which include prospective observational studies, have not generally indicated a drug-associated risk of major birth defects with interferon beta-1b during pregnancy.
Administration of interferon beta-1b to monkeys during gestation resulted in increased embryo-fetal death at or above exposures greater than 3 times the human therapeutic dose (see Animal Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Human Data
The majority of the observational studies reporting on pregnancies exposed to interferon beta-1b did not identify an association between the use of interferon beta-1b during pregnancy and an increased risk of major birth defects.
Animal Data
When interferon beta-1b (doses ranging from 0.028 to 0.42 mg/kg/day) was administered to pregnant rhesus monkeys throughout the period of organogenesis (gestation days 20 to 70), a dose-related increase in the incidence of abortion was observed. The low-effect dose is approximately 3 times the recommended human dose of 0.25 mg on a body surface area (mg/m2) basis. A no-effect dose for embryo-fetal developmental toxicity in rhesus monkeys was not established.
8.2 Lactation
Risk Summary
There are no data on the presence of interferon beta-1b in human milk, the effects on the breastfed infant, or the effects of the drug on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EXTAVIA and any potential adverse effects on the breastfed child from EXTAVIA or from the underlying maternal condition.
12. Extavia - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of EXTAVIA (interferon beta-1b) in patients with MS is unknown.
12.2 Pharmacodynamics
Interferons (IFNs) are a family of naturally occurring proteins, produced by eukaryotic cells in response to viral infection and other biologic agents. Three major types of interferons have been defined: type I (IFN-alpha, beta, epsilon, kappa, and omega), type II (IFN-gamma) and type III (IFN-lambda). Interferon-beta is a member of the type I subset of interferons. The type I interferons have considerably overlapping but also distinct biologic activities. The bioactivities of all IFNs, including IFN-beta, are induced via their binding to specific receptors on the membranes of human cells. Differences in the bioactivities induced by the three major subtypes of IFNs likely reflect differences in the signal transduction pathways induced by signaling through their cognate receptors.
Interferon beta-1b receptor binding induces the expression of proteins that are responsible for the pleiotropic bioactivities of interferon beta-1b. A number of these proteins (including neopterin, β2-microglobulin, MxA protein, and IL-10) have been measured in blood fractions from interferon beta-1b-treated patients and interferon beta-1b-treated healthy volunteers. Immunomodulatory effects of interferon beta-1b include the enhancement of suppressor T-cell activity, reduction of pro-inflammatory cytokine production, down-regulation of antigen presentation, and inhibition of lymphocyte trafficking into the central nervous system. It is not known if these effects play an important role in the observed clinical activity of interferon beta-1b in MS.
12.3 Pharmacokinetics
Because serum concentrations of interferon beta-1b are low or not detectable following subcutaneous administration of 0.25 mg or less of interferon beta-1b, pharmacokinetic information in patients with MS receiving the recommended dose of interferon beta-1b is not available.
Following single and multiple daily subcutaneous administrations of 0.5 mg interferon beta-1b to healthy volunteers (N = 12), serum interferon beta-1b concentrations were generally below 100 units/mL. Peak serum interferon beta-1b concentrations occurred between 1 to 8 hours, with a mean peak serum interferon concentration of 40 units/mL. Bioavailability, based on a total dose of 0.5 mg interferon beta-1b given as two subcutaneous injections at different sites, was approximately 50%.
After intravenous administration of interferon beta-1b (0.006 mg to 2 mg), similar pharmacokinetic profiles were obtained from healthy volunteers (N = 12) and from patients with diseases other than MS (N = 142). In patients receiving single intravenous doses up to 2 mg, increases in serum concentrations were dose proportional. Mean serum clearance values ranged from 9.4 mL/min•kg-1 to 28.9 mL/min•kg-1 and were independent of dose. Mean terminal elimination half-life values ranged from 8 minutes to 4.3 hours, and mean steady-state volume of distribution values ranged from 0.25 L/kg to 2.88 L/kg. Three-times-a-week intravenous dosing for two weeks resulted in no accumulation of interferon beta-1b in sera of patients. Pharmacokinetic parameters after single and multiple intravenous doses of interferon beta-1b were comparable.
Following every-other-day subcutaneous administration of 0.25 mg interferon beta-1b in healthy volunteers, biologic response marker levels (neopterin, β2-microglobulin, MxA protein, and the immunosuppressive cytokine, IL-10) increased significantly above baseline 6 to 12 hours after the first interferon beta-1b dose. Biologic response marker levels peaked between 40 and 124 hours and remained elevated above baseline throughout the seven-day (168-hour) study. The relationship between serum interferon beta-1b levels or induced biologic response marker levels and the clinical effects of interferon beta-1b in MS is unknown.
Drug Interaction Studies
No formal drug interaction studies have been conducted with interferon beta-1b.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Interferon beta-1b has not been tested for its carcinogenic potential in animals.
Mutagenesis
Interferon beta-1b was not genotoxic in the in vitro bacterial reverse mutation assay or the in vitro chromosomal aberration assay in human peripheral blood lymphocytes. Interferon beta-1b treatment of mouse BALBc-3T3 cells did not result in increased transformation frequency in an in vitro model of tumor transformation.
Impairment of Fertility
Administration of interferon beta-1b (doses of up to 0.33 mg/kg/day) to normally cycling female rhesus monkeys had no apparent adverse effects on either menstrual cycle duration or associated hormonal profiles (progesterone and estradiol) when administered over three consecutive menstrual cycles. The highest dose tested is approximately 30 times the recommended human dose of 0.25 mg on a body surface area (mg/m2) basis. The potential for other effects on fertility or reproductive performance was not evaluated.
16. How is Extavia supplied
16.2 Stability and Storage
EXTAVIA and the diluent are for single-use only. Discard unused portions. The reconstituted product contains no preservative. Store EXTAVIA vials at room temperature 20°C to 25°C (68°F to 77°F). Excursions are permitted between 15°C and 30°C (59°F and 86°F) for up to 3 months [see USP Controlled Room Temperature]. After reconstitution, if not used immediately, refrigerate the reconstituted solution and use within three hours. Do not freeze.
| EXTAVIA
interferon beta-1b kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |