Drug Detail:Fetroja (Cefiderocol [ sef-i-der-oh-kol ])
Drug Class: Other cephalosporins
Highlights of Prescribing Information
FETROJA (cefiderocol) for injection, for intravenous use
Initial U.S. Approval: 2019
Recent Major Changes
| Dosage and Administration (2) | 11/2021 |
Indications and Usage for Fetroja
FETROJA is a cephalosporin antibacterial indicated in patients 18 years of age or older for the treatment of the following infections caused by susceptible Gram-negative microorganisms:
- Complicated Urinary Tract Infections (cUTI), including Pyelonephritis (1.1)
- Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP) (1.2)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of FETROJA and other antibacterial drugs, FETROJA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.3)
Fetroja Dosage and Administration
- Administer 2 grams of FETROJA for injection every 8 hours by intravenous (IV) infusion over 3 hours in patients with creatinine clearance (CLcr) 60 to 119 mL/min. (2.1)
- Dose adjustments are required for patients with CLcr less than 60 mL/min, (including patients receiving intermittent hemodialysis (HD) or continuous renal replacement therapy (CRRT)), and for patients with CLcr 120 mL/min or greater. (2.2)
- See full prescribing information for instructions on preparation of FETROJA doses. (2.3)
- See full prescribing information for drug compatibilities. (2.4)
Dosage Forms and Strengths
For injection: 1 gram of cefiderocol as a lyophilized powder for reconstitution in single-dose vials. (3)
Contraindications
FETROJA is contraindicated in patients with a known history of severe hypersensitivity to cefiderocol and other beta-lactam antibacterial drugs or other components of FETROJA. (4)
Warnings and Precautions
- Increase in All-Cause Mortality in Patients with Carbapenem-Resistant Gram-Negative Bacterial Infections: An increase in all-cause mortality was observed in FETROJA-treated patients compared to those treated with best available therapy (BAT). Closely monitor the clinical response to therapy in patients with cUTI and HABP/VABP. (5.1)
- Hypersensitivity Reactions: Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving beta-lactam antibacterial drugs. Hypersensitivity was observed with FETROJA. Cross-hypersensitivity may occur in patients with a history of penicillin allergy. If an allergic reaction occurs, discontinue FETROJA. (5.2)
- Clostridioides difficile-associated Diarrhea (CDAD): CDAD has been reported with nearly all systemic antibacterial agents, including FETROJA. Evaluate if diarrhea occurs. (5.3)
- Seizures and Other Central Nervous System (CNS) Adverse Reactions: CNS adverse reactions such as seizures have been reported with FETROJA. If focal tremors, myoclonus, or seizures occur, evaluate patients to determine whether FETROJA should be discontinued. (5.4)
Adverse Reactions/Side Effects
- cUTI: The most frequently occurring adverse reactions in greater than or equal to 2% of cUTI patients treated with FETROJA were diarrhea, infusion site reactions, constipation, rash, candidiasis, cough, elevations in liver tests, headache, hypokalemia, nausea, and vomiting. (6.1)
- HABP/VABP: The most frequently occurring adverse reactions in greater than or equal to 4% of HABP/VABP patients treated with FETROJA were elevations in liver tests, hypokalemia, diarrhea, hypomagnesemia, and atrial fibrillation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Shionogi Inc. at 1-800-849-9707 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Use alternate testing methods to confirm positive results of dipstick tests (urine protein, ketones, or occult blood). (7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2021
Full Prescribing Information
1. Indications and Usage for Fetroja
1.1 Complicated Urinary Tract Infections (cUTIs), Including Pyelonephritis
FETROJA® is indicated in patients 18 years of age or older for the treatment of complicated urinary tract infections (cUTIs), including pyelonephritis caused by the following susceptible Gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and Enterobacter cloacae complex [see Clinical Studies (14.1)].
1.2 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP)
FETROJA is indicated in patients 18 years of age or older for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia, caused by the following susceptible Gram-negative microorganisms: Acinetobacter baumannii complex, Escherichia coli, Enterobacter cloacae complex, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Serratia marcescens [see Clinical Studies (14.2)].
1.3 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of FETROJA and other antibacterial drugs, FETROJA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2. Fetroja Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of FETROJA is 2 grams administered every 8 hours by intravenous (IV) infusion over 3 hours in adults with a creatinine clearance (CLcr) of 60 to 119 mL/min.
Dosage adjustment of FETROJA is recommended for patients with CLcr less than 60 mL/min, including patients receiving intermittent hemodialysis (HD) or continuous renal replacement therapy (CRRT), and for patients with CLcr 120 mL/min or greater [see Dosage and Administration (2.2)]. The recommended duration of treatment with FETROJA is 7 to 14 days. The duration of therapy should be guided by the patient's clinical status.
2.2 Dosage Adjustments in Patients with CLcr Less Than 60 mL/min (Including Patients Undergoing Intermittent HD or CRRT), and CLcr 120 mL/min or Greater
Dosage Adjustments in Patients Receiving CRRT
For patients receiving CRRT, including continuous venovenous hemofiltration (CVVH), continuous venovenous hemodialysis (CVVHD), and continuous venovenous hemodiafiltration (CVVHDF), the dosage of FETROJA should be based on the effluent flow rate in CRRT (see Table 2). These recommendations are intended to provide initial dosing in patients receiving CRRT. Dosing regimens may need to be tailored based on residual renal function and patient's clinical status [see Use in Specific Populations (8.6)].
| Effluent Flow Rate * | Recommended Dosage of FETROJA |
|---|---|
| CRRT = continuous renal replacement therapy. | |
|
|
| 2 L/hr or less | 1.5 grams every 12 hours |
| 2.1 to 3 L/hr | 2 grams every 12 hours |
| 3.1 to 4 L/hr | 1.5 grams every 8 hours |
| 4.1 L/hr or greater | 2 grams every 8 hours |
2.3 Preparation of FETROJA Solution for Administration
FETROJA is supplied as a sterile, lyophilized powder that must be reconstituted and subsequently diluted using aseptic technique prior to intravenous infusion.
Preparation of Doses
Reconstitute the powder for injection in the FETROJA vial with 10 mL of either 0.9% sodium chloride injection, USP or 5% dextrose injection, USP and gently shake to dissolve. Allow the vial(s) to stand until the foaming generated on the surface has disappeared (typically within 2 minutes). The reconstituted solution will have a final volume of approximately 11.2 mL and concentration of 0.089 gram/mL. The reconstituted solution is for intravenous infusion only after dilution in an appropriate infusion solution.
To prepare the required doses, withdraw the appropriate volume of reconstituted solution from the vial according to Table 3 below. Add the withdrawn volume to a 100 mL infusion bag containing 0.9% sodium chloride injection, USP or 5% dextrose injection, USP [see Dosage and Administration (2.4)].
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. FETROJA infusions are clear, colorless solutions. Discard any unused FETROJA solution in the vial (see Table 3).
| FETROJA Dose | Number of 1-gram FETROJA Vials to be Reconstituted | Volume to Withdraw from Reconstituted Vial(s) | Total Volume of FETROJA Reconstituted Solution for Further Dilution into a 100 mL Infusion Bag |
|---|---|---|---|
| 2 grams | 2 vials | 11.2 mL (entire contents) of each vial | 22.4 mL |
| 1.5 grams | 2 vials | 11.2 mL (entire contents) of first vial AND 5.6 mL from second vial | 16.8 mL |
| 1 gram | 1 vial | 11.2 mL (entire contents) | 11.2 mL |
| 0.75 gram | 1 vial | 8.4 mL | 8.4 mL |
2.4 Drug Compatibility
FETROJA solution for administration is compatible with:
- 0.9% sodium chloride injection, USP
- 5% dextrose injection, USP
The compatibility of FETROJA solution for administration with solutions containing other drugs or other diluents has not been established.
3. Dosage Forms and Strengths
FETROJA 1 gram for injection is supplied as a white to off-white, sterile, lyophilized powder for reconstitution in single-dose, clear glass vials; each vial contains 1 gram of cefiderocol.
4. Contraindications
FETROJA is contraindicated in patients with a known history of severe hypersensitivity to cefiderocol or other beta-lactam antibacterial drugs, or any other component of FETROJA [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
5. Warnings and Precautions
5.1 Increase in All-Cause Mortality in Patients with Carbapenem-Resistant Gram-Negative Bacterial Infections
An increase in all-cause mortality was observed in patients treated with FETROJA as compared to best available therapy (BAT) in a multinational, randomized, open-label trial in critically ill patients with carbapenem-resistant Gram-negative bacterial infections (NCT02714595). Patients with nosocomial pneumonia, bloodstream infections, sepsis, or cUTI were included in the trial. BAT regimens varied according to local practices and consisted of 1 to 3 antibacterial drugs with activity against Gram-negative bacteria. Most of the BAT regimens contained colistin.
The increase in all-cause mortality occurred in patients treated for nosocomial pneumonia, bloodstream infections, or sepsis. The 28-Day all-cause mortality was higher in patients treated with FETROJA than in patients treated with BAT [25/101 (24.8%) vs. 9/49 (18.4%), treatment difference 6.4%, 95% CI (-8.6, 19.2)]. All-cause mortality remained higher in patients treated with FETROJA than in patients treated with BAT through Day 49 [34/101 (33.7%) vs. 10/49 (20.4%), treatment difference 13.3%, 95% CI (-2.5, 26.9)]. Generally, deaths were in patients with infections caused by Gram-negative organisms, including non-fermenters such as Acinetobacter baumannii complex, Stenotrophomonas maltophilia, and Pseudomonas aeruginosa, and were the result of worsening or complications of infection, or underlying comorbidities. The cause of the increase in mortality has not been established.
Closely monitor the clinical response to therapy in patients with cUTI and HABP/VABP.
5.2 Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions and serious skin reactions have been reported in patients receiving beta-lactam antibacterial drugs. Hypersensitivity was observed in FETROJA-treated patients in clinical trials [see Adverse Reactions (6.1)]. These reactions are more likely to occur in individuals with a history of beta-lactam hypersensitivity and/or a history of sensitivity to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins.
Before therapy with FETROJA is instituted, inquire about previous hypersensitivity reactions to cephalosporins, penicillins, or other beta-lactam antibacterial drugs. Discontinue FETROJA if an allergic reaction occurs.
5.3 Clostridioides difficile-associated Diarrhea (CDAD)
Clostridioides difficile-associated diarrhea (CDAD) has been reported for nearly all systemic antibacterial agents, including FETROJA. CDAD may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, antibacterial drugs not directed against C. difficile may need to be discontinued. Manage fluid and electrolyte levels as appropriate, supplement protein intake, monitor antibacterial treatment of C. difficile, and institute surgical evaluation as clinically indicated.
5.4 Seizures and Other Central Nervous System (CNS) Adverse Reactions
Cephalosporins, including FETROJA, have been implicated in triggering seizures [see Adverse Reactions (6.1)]. Nonconvulsive status epilepticus (NCSE), encephalopathy, coma, asterixis, neuromuscular excitability, and myoclonia have been reported with cephalosporins particularly in patients with a history of epilepsy and/or when recommended dosages of cephalosporins were exceeded due to renal impairment. Adjust FETROJA dosing based on creatinine clearance [see Dosage and Administration (2.2)]. Anticonvulsant therapy should be continued in patients with known seizure disorders. If CNS adverse reactions including seizures occur, patients should undergo a neurological evaluation to determine whether FETROJA should be discontinued.
5.5 Development of Drug-Resistant Bacteria
Prescribing FETROJA in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria [see Indications and Usage (1.3)].
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described in greater detail in the Warnings and Precautions section:
- Increase in All-Cause Mortality in Patients with Carbapenem-Resistant Gram-Negative Bacterial Infections [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Clostridioides difficile-associated Diarrhea (CDAD) [see Warnings and Precautions (5.3)]
- Seizures and Other Central Nervous System Adverse Reactions [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of FETROJA in pediatric patients younger than 18 years of age have not been established.
10. Overdosage
There is no information on clinical signs and symptoms associated with an overdose of FETROJA. Patients who receive doses greater than the recommended dose regimen and have unexpected adverse reactions possibly associated with FETROJA should be carefully observed and given supportive treatment, and discontinuation or interruption of treatment should be considered.
Approximately 60% of cefiderocol is removed by a 3- to 4-hour hemodialysis session [see Clinical Pharmacology (12.3)].
11. Fetroja Description
FETROJA is a cephalosporin antibacterial drug product consisting of cefiderocol sulfate tosylate for intravenous infusion. Cefiderocol functions as a siderophore [see Microbiology (12.4)].
The chemical name of cefiderocol sulfate tosylate is Tris[(6R,7R)-7-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-{[(2-carboxypropan-2-yl)oxy]imino}acetamido]-3-({1-[2-(2-chloro-3,4-dihydroxybenzamido)ethyl]pyrrolidin-1-ium-1-yl}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate] tetrakis(4-methylbenzenesulfonate) monosulfate hydrate, and the molecular weight is 3043.50 (anhydrous). The molecular formula is 3C30H34ClN7O10S2∙4C7H8O3S∙H2SO4∙xH2O.
| Figure 1 Chemical Structure of Cefiderocol Sulfate Tosylate |
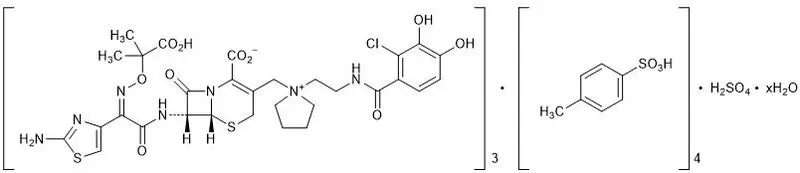 |
FETROJA for injection is a white to off-white, sterile, lyophilized powder formulated with 1 gram of cefiderocol (equivalent to 1.6 grams of cefiderocol sulfate tosylate), sucrose (900 mg), sodium chloride (216 mg), and sodium hydroxide to adjust pH. The sodium content is approximately 176 mg/vial. The pH of the reconstituted solution of 1 gram cefiderocol (1 vial) dissolved in 10 mL water is 5.2 to 5.8.
12. Fetroja - Clinical Pharmacology
12.2 Pharmacodynamics
The percent time of dosing interval that unbound plasma concentrations of cefiderocol exceed the minimum inhibitory concentration (MIC) against the infecting organism best correlates with antibacterial activity in neutropenic murine thigh and lung infection models with E. coli, K. pneumoniae, P. aeruginosa, A. baumannii, and S. maltophilia. Compared to a 1-hour infusion, a 3-hour infusion increased the percent time of dosing interval that unbound plasma concentrations of cefiderocol exceed the MIC. The in vivo animal pneumonia studies showed that the antibacterial activity of cefiderocol was greater at the human equivalent dosing regimen of 3-hour infusion compared to that of 1-hour infusion.
12.3 Pharmacokinetics
Cefiderocol exposures (Cmax and daily AUC) in cUTI patients, HABP/VABP patients, and healthy volunteers are summarized in Table 6. Cefiderocol Cmax and AUC increased proportionally with dose.
| PK Parameters | cUTI Patients*
(N = 21) | HABP/VABP Patients*
(N = 146) | Healthy Volunteers†
(N = 43) |
|---|---|---|---|
| Cmax = maximum concentration. | |||
| AUC0-24 hr = area under the concentration time curve from 0 to 24 hours. | |||
|
|||
| Cmax (mg/L) | 115 (±57) | 111 (±56) | 91.4 (±17.9) |
| AUC0-24 hr (mg∙hr/L) | 1944 (±1097) | 1773 (±990) | 1175 (±203) |
14. Clinical Studies
14.1 Complicated Urinary Tract Infections, Including Pyelonephritis
A total of 448 adults hospitalized with cUTI (including pyelonephritis) were randomized in a 2:1 ratio and received study medications in a multinational, double-blind trial (Trial 1) (NCT02321800) comparing FETROJA 2 grams intravenously (IV) every 8 hours (infused over 1 hour) to imipenem/cilastatin 1gram/1gram IV every 8 hours (infused over 1 hour) for 7 to 14 days. No switch from IV to oral antibacterial therapy was permitted.
Efficacy was assessed as a composite of microbiological eradication and clinical cure at the Test-of-Cure visit (TOC) in the microbiological intent-to-treat (Micro-ITT) population, which included all patients who received at least a single dose of study medication and had at least one baseline Gram-negative uropathogen. Other efficacy endpoints included the microbiological eradication rate and the clinical response rate at TOC in the Micro-ITT population.
The Micro-ITT population consisted of 371 patients of whom 25% had cUTI with pyelonephritis, 48% had cUTI without pyelonephritis, and 27% had acute uncomplicated pyelonephritis. Complicating conditions included obstructive uropathy, catheterization, and renal stones. The median age was 66 years, with 24% of patients over the age of 75 years, and 55% of the population were female. The median duration of therapy in both treatment groups was 9 days (range 1-14 days). Of the 371 patients, 32% had CLcr > 50-80 mL/min, 17% had CLcr 30-50 mL/min, and 3% had CLcr < 30 mL/min at baseline. Concomitant Gram-negative bacteremia was identified in 7% of patients. In the Micro-ITT population, the most common baseline pathogens were E. coli and K. pneumoniae.
Table 8 provides the results of a composite of microbiological eradication (all Gram-negative uropathogens found at baseline at ≥ 105 CFU/mL reduced to < 104 CFU/mL) and clinical response (resolution or improvement of cUTI symptoms and no new symptoms assessed by the investigator) at the TOC visit, 7+/-2 days after the last dose of study drug. The response rates for the composite endpoint of microbiological eradication and clinical response at the TOC visit were higher in the FETROJA arm compared with imipenem/cilastatin, as shown in Table 8. Clinical response rates at the TOC visit were similar between FETROJA and imipenem/cilastatin. Most patients with microbiological failure at the TOC visit in either treatment arm did not require further antibacterial drug treatment. Subgroup analyses examining composite outcomes by baseline pathogen are shown in Table 9 and demonstrated responses consistent with the overall population. Subgroup analyses examining outcomes by age, gender, and/or outcomes in patients with renal impairment, concomitant bacteremia, complicated UTI with or without pyelonephritis, or acute uncomplicated pyelonephritis demonstrated responses were consistent with the overall population.
| Study Endpoint | FETROJA n/N (%) | Imipenem/Cilastatin n/N (%) | Treatment Difference (95% CI)* |
|---|---|---|---|
| CI = confidence interval; Micro-ITT = microbiological intent-to-treat; TOC = Test of Cure. | |||
|
|||
| Composite response at TOC | 183/252 (72.6%) | 65/119 (54.6%) | 18.6 (8.2, 28.9) |
| Microbiologic response TOC | 184/252 (73.0%) | 67/119 (56.3%) | 17.3 (6.9, 27.6) |
| Clinical response TOC | 226/252 (89.7%) | 104/119 (87.4%) | 2.4 (-4.7, 9.4) |
| Baseline Pathogen Subgroup | FETROJA n/N (%) | Imipenem/Cilastatin n/N (%) |
|---|---|---|
|
||
| Escherichia coli | 113/152 (74.3) | 45/79 (57.0) |
| Klebsiella pneumoniae | 36/48 (75.0) | 12/25 (48.0) |
| Proteus mirabilis | 13/17 (76.5) | 0/2 (0.0) |
| Pseudomonas aeruginosa | 8/18 (44.4) | 3/5 (60.0) |
| Enterobacter cloacae complex | 8/13 (61.5) | 3/3 (100.0) |
In the FETROJA treatment group, 61 (24.2%) bacterial isolates were ESBL producers compared with 32 (26.9%) in the imipenem/cilastatin group. The composite response rate of patients with these ESBL isolates at the TOC visit was consistent with the overall results.
14.2 Hospital-acquired Bacterial Pneumonia and Ventilator-associated Bacterial Pneumonia (HABP/VABP)
A total of 298 hospitalized adults with HABP/VABP received study medications in a multicenter, randomized, double-blind trial (Trial 2) (NCT03032380) comparing FETROJA 2 grams administered intravenously every 8 hours as a 3-hour infusion to meropenem (2 grams every 8 hours infused over 3 hours). Dosing was adjusted for renal function. Patients in both treatment arms received linezolid 600 mg every 12 hours for at least 5 days for empiric treatment of Gram-positive organisms. The trial protocol permitted administration of potentially active prior antibacterial therapy for no more than 24 hours within 72 hours prior to randomization and disallowed systemic concomitant antibacterial therapy until the Test-of-Cure visit (TOC, 7 days after end of treatment). The analysis population was the modified intent-to-treat (mITT) population, which included all randomized patients who received study medication and had evidence of bacterial pneumonia, except those with only anaerobic or Gram-positive aerobic infections.
Of the 292 patients in the mITT population, the median age was 67 years, and 58% of the population was 65 years of age and older, with 29% of the population 75 years of age and older. The majority of patients were male (68%), White (69%), and were from Europe (67%). Approximately 4% (11/292) were from the United States. The median baseline APACHE II score was 15, and 29% of patients had a baseline APACHE II score of greater than or equal to 20. At randomization, 68% of patients were in the ICU, and 60% were mechanically ventilated. 60% of patients had CLcr less than or equal to 80 mL/min at baseline; among these, 34% had CLcr less than or equal to 50 mL/min, and 14% had a CLcr less than 30 mL/min. Augmented renal clearance (CLcr greater than 120 mL/min) was present in 16% of patients. Gram-negative bacteremia was present at baseline in 6% of patients. In both treatment groups, most patients (70%) received between 7 and 14 days of study medication and 18% between 15 and 21 days.
Table 10 shows the Day 14 and Day 28 all-cause mortality rates, as well as clinical cure at the TOC visit. FETROJA was noninferior to meropenem with regard to the primary efficacy endpoint (Day 14 all-cause mortality in the mITT population). Clinical cure was defined as resolution or substantial improvement in signs and symptoms associated with pneumonia, such that no additional antibacterial therapy was required for the treatment of the current infection through the TOC visit.
| Endpoint | FETROJA n/N (%) | Meropenem n/N (%) | Treatment Difference*
(95% CI) |
|---|---|---|---|
| CI = confidence interval; TOC = Test of Cure. | |||
|
|||
| Day 14 All-cause Mortality | 18/145 (12.4) | 18/147 (12.2) | 0.2 (-7.2, 7.7) |
| Day 28 All-cause Mortality | 32/145 (22.1) | 31/147 (21.1) | 1.1 (-8.2, 10.4) |
| Clinical Cure at TOC | 94/145 (64.8) | 98/147 (66.7) | -2.0 (-12.5, 8.5) |
The Day 14 and Day 28 all-cause mortality rates by pathogen in patients in the mITT population who had a baseline LRT pathogen that was susceptible to meropenem are shown in Table 11; the clinical outcome at the TOC visit is shown in Table 12. There were 51 patients with A. baumannii complex at baseline, of which 17 (33.3%) patients had isolates susceptible to meropenem (MIC ≤ 8 mcg/mL, based on meropenem 2 grams every 8 hours). Among 51 patients with A. baumannii complex at baseline, all-cause mortality at Day 14 was 5/26 (19.2%) in FETROJA and 4/25 (16.0%) in the meropenem treatment group and at Day 28 was 9/26 (34.6%) in FETROJA and 6/25 (24.0%) in the meropenem treatment group. The clinical cure rates at the TOC visit were 14/26 (53.8%) in the FETROJA and 15/25 (60.0%) in the meropenem treatment group.
| Baseline Pathogen | Day 14 All-cause Mortality | Day 28 All-cause Mortality | ||
|---|---|---|---|---|
| FETROJA n/N (%) | Meropenem n/N (%) | FETROJA n/N (%) | Meropenem n/N (%) |
|
| Each cell excludes subjects in whom baseline pathogen had meropenem MIC > 8 mcg/mL or where MIC was unknown. Subjects with unknown survival status were considered deaths. |
||||
|
||||
| Klebsiella pneumoniae | 4/38 (10.5) | 4/36 (11.1) | 8/38 (21.1) | 9/36 (25.0) |
| Pseudomonas aeruginosa | 2/20 (10.0) | 4/17 (23.5) | 2/20 (10.0) | 5/17 (29.4) |
| Acinetobacter baumannii complex† | 1/8 (12.5) | 0/9 (0.0) | 3/8 (37.5) | 0/9 (0.0) |
| Escherichia coli | 3/18 (16.7) | 3/21 (14.3) | 5/18 (27.8) | 4/21 (19.0) |
| Other Enterobacterales‡ | 2/16 (12.5) | 2/14 (14.3) | 4/16 (25.0) | 3/14 (21.4) |
| Baseline Pathogen | Clinical Cure | |
|---|---|---|
| FETROJA n/N (%) | Meropenem n/N (%) |
|
| Each cell excludes subjects whose pathogen-specific meropenem MIC was > 8 mcg/mL or where MIC was unknown. | ||
|
||
| Klebsiella pneumoniae | 24/38 (63.2) | 23/36 (63.9) |
| Pseudomonas aeruginosa | 13/20 (65.0) | 13/17 (76.5) |
| Acinetobacter baumannii complex† | 6/8 (75.0) | 7/9 (77.8) |
| Escherichia coli | 12/18 (66.7) | 13/21 (61.9) |
| Other Enterobacterales‡ | 10/16 (62.5) | 8/14 (57.1) |
In the FETROJA treatment group, 45 (31%) patients had ESBL-producing bacterial isolates compared with 42 (28.6%) patients in the meropenem treatment group. All-cause mortality at Day 14 and Day 28 of patients with these ESBL-producing bacterial isolates was consistent with the overall results.
16. How is Fetroja supplied
16.1 How Supplied
FETROJA 1 gram (cefiderocol) for injection is supplied as a white to off-white sterile lyophilized powder for reconstitution in single-dose, clear glass vials (NDC 59630-266-01) sealed with a rubber stopper (not made with natural rubber latex) and an aluminum seal with flip-off cap. Each vial is supplied in cartons containing 10 single-dose vials.
| NDC 59630-266-10 | FETROJA (cefiderocol) 1 gram/vial, 10 vials/carton |
| FETROJA
cefiderocol sulfate tosylate injection, powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Shionogi Inc. (098241610) |





