Drug Detail:Glyset (Miglitol [ mig-li-tol ])
Drug Class: Alpha-glucosidase inhibitors
Glyset - Clinical Pharmacology
Miglitol is a desoxynojirimycin derivative that delays the digestion of ingested carbohydrates, thereby resulting in a smaller rise in blood glucose concentration following meals. As a consequence of plasma glucose reduction, GLYSET Tablets reduce levels of glycosylated hemoglobin in patients with Type II (non-insulin-dependent) diabetes mellitus. Systemic nonenzymatic protein glycosylation, as reflected by levels of glycosylated hemoglobin, is a function of average blood glucose concentration over time.
Clinical Studies
Clinical Experience in Non-Insulin-Dependent Diabetes Mellitus (NIDDM) Patients on Dietary Treatment Only
GLYSET Tablets were evaluated in two U.S. and three non-U.S. controlled, fixed-dose, monotherapy studies, in which 735 patients treated with GLYSET were evaluated for efficacy analyses (see Table 1).
In Study 1, a 1-year study in which GLYSET was evaluated as monotherapy and also as combination therapy, there was a statistically significantly smaller increase in mean glycosylated hemoglobin (HbA1c) over time in the miglitol 50 mg 3 times daily monotherapy arm compared to placebo. Significant reductions in mean fasting and postprandial plasma glucose levels and in mean postprandial insulin levels were observed in patients treated with GLYSET compared with the placebo group.
In Study 2, a 14-week study, there was a significant decrease in HbA1c in patients receiving GLYSET 50 mg 3 times daily or 100 mg 3 times daily compared to placebo. In addition, there were significant reductions in postprandial plasma glucose and postprandial serum insulin levels compared to placebo.
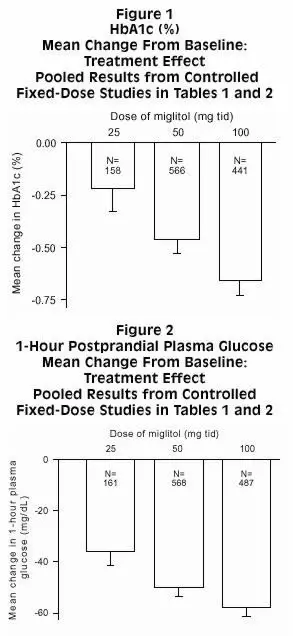
Study 3, was a 6-month dose-ranging trial evaluating GLYSET at doses from 25 mg 3 times daily, to 200 mg 3 times daily. GLYSET produced a greater reduction in HbA1c than placebo at all doses, although the effect was statistically significant at the 100 mg 3 times daily and 200 mg 3 times daily. In addition, all doses of GLYSET produced significant reductions in postprandial plasma glucose and postprandial insulin levels compared to placebo.
Studies 4 and 5 were 6-month studies evaluating GLYSET at 50 and 100 mg 3 times daily, and 100 mg 3 times daily, respectively. As compared to placebo, GLYSET produced reductions in HbA1c, as well as a significant reduction in postprandial plasma glucose in both studies at the doses employed.
| HbA1c (%) | 1-hour Postprandial Glucose (mg/dL) |
||||
|---|---|---|---|---|---|
| Study | Treatment | Mean Change from Baseline* | Treatment Effect† | Mean Change from Baseline | Treatment Effect† |
|
|||||
| 1 (U.S.) | Placebo | +0.71 | --- | +24 | --- |
| GLYSET 50 mg 3 times daily | +0.13 | -0.58‡ | -39 | -63‡ | |
| 2 (U.S.) | Placebo | +0.47 | --- | +15 | --- |
| GLYSET 50 mg 3 times daily | -0.22 | -0.69‡ | -52 | -67‡ | |
| GLYSET 100 mg 3 times daily | -0.28 | -0.75‡ | -59 | -74‡ | |
| 3 (non-U.S.) | Placebo | +0.18 | --- | +2 | --- |
| GLYSET 25 mg 3 times daily | -0.08 | -0.26 | -33 | -35‡ | |
| GLYSET 50 mg 3 times daily | -0.22 | -0.40 | -45 | -47‡ | |
| GLYSET 100 mg 3 times daily | -0.63 | -0.81‡ | -62 | -64‡ | |
| GLYSET 200 mg 3 times daily § | -0.84 | -1.02‡ | -85 | -87‡ | |
| 4 (non-U.S.) | Placebo | +0.01 | --- | +8 | --- |
| GLYSET 50 mg 3 times daily | -0.35 | -0.36‡ | -20 | -28‡ | |
| GLYSET 100 mg 3 times daily | -0.57 | -0.58‡ | -25 | -33‡ | |
| 5 (non-U.S.) | Placebo | +0.32 | --- | +17 | --- |
| GLYSET 100 mg 3 times daily | -0.43 | -0.75‡ | -38 | -55‡ | |
Clinical Experience in NIDDM Patients Receiving Sulfonylureas
GLYSET was studied as adjunctive therapy to a background of maximal or near-maximal sulfonylurea (SFU) treatment in three large, double-blind, randomized studies (two U.S. and one non-U.S.) in which 471 patients treated with GLYSET were evaluated for efficacy (see Table 2).
Study 6 included patients under treatment with maximal doses of SFU at entry. At the end of this 14-week study, the mean treatment effects on glycosylated hemoglobin (HbA1c) were -0.82% and -0.74% for patients receiving GLYSET 50 mg 3 times daily plus SFU, and GLYSET 100 mg 3 times daily plus SFU, respectively.
Study 7 was a 1-year study in which GLYSET at 25, 50 or 100 mg 3 times daily was added to a maximal dose of glyburide (10 mg twice daily). At the end of this study, the mean treatment effects on HbA1c of GLYSET when added to maximum glyburide therapy were -0.30%, -0.62%, and -0.73% with 25, 50 and 100 mg 3 times daily dosages of GLYSET, respectively.
In Study 8, the addition of GLYSET 100 mg 3 times daily to a background of treatment with glyburide produced an additional mean treatment effect on HbA1c of -0.66%.
| HbA1c (%) | 1-hour Postprandial Glucose (mg/dL) |
||||
|---|---|---|---|---|---|
| Study | Treatment | Mean Change from Baseline* | Treatment Effect† | Mean Change from Baseline | Treatment Effect† |
|
|||||
| 6 (U.S.) | Placebo + SFU | +0.33 | --- | -1 | --- |
| GLYSET 50 mg 3 times daily + SFU | -0.49 | -0.82‡ | -69 | -68‡ | |
| GLYSET 100 mg 3 times daily + SFU | -0.41 | -0.74‡ | -73 | -72‡ | |
| 7 (U.S.) | Placebo + SFU | +1.01 | --- | 48 | --- |
| GLYSET 25 mg 3 times daily + SFU | +0.71 | -0.30 | -2 | -50‡ | |
| GLYSET 50 mg 3 times daily + SFU | +0.39 | -0.62‡ | -13 | -61‡ | |
| GLYSET 100 mg 3 times daily + SFU | +0.28 | -0.73‡ | -33 | -81‡ | |
| 8 (non-U.S.) | Placebo + SFU | +0.16 | --- | +10 | --- |
| GLYSET 100 mg 3 times daily + SFU | -0.50 | -0.66‡ | -36 | -46‡ | |
Glyset Dosage and Administration
There is no fixed dosage regimen for the management of diabetes mellitus with GLYSET Tablets or any other pharmacologic agent. Dosage of GLYSET must be individualized on the basis of both effectiveness and tolerance while not exceeding the maximum recommended dosage of 100 mg 3 times daily. GLYSET should be taken three times daily at the start of each main meal. GLYSET should be started at 25 mg, and the dosage gradually increased both to reduce gastrointestinal adverse effects and to permit identification of the minimum dose required for adequate glycemic control of the patient.
During treatment initiation and dose titration one-hour postprandial plasma glucose may be used to determine the therapeutic response to GLYSET and identify the minimum effective dose for the patient. Thereafter, glycosylated hemoglobin should be measured at intervals of approximately 3 months. The therapeutic goal should be to decrease both postprandial plasma glucose and glycosylated hemoglobin levels to normal or near normal by using the lowest effective dose of GLYSET, either as monotherapy or in combination with a sulfonylurea.
Initial Dosage
The recommended starting dosage of GLYSET is 25 mg, given orally three times daily at the start of each main meal. However, some patients may benefit by starting at 25 mg once daily to minimize gastrointestinal adverse effects, and gradually increasing the frequency of administration to 3 times daily.
| GLYSET
miglitol tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| GLYSET
miglitol tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| GLYSET
miglitol tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | ANALYSIS(0009-5012, 0009-5013, 0009-5014) , MANUFACTURE(0009-5012, 0009-5013, 0009-5014) , API MANUFACTURE(0009-5012, 0009-5013, 0009-5014) , PACK(0009-5012, 0009-5013, 0009-5014) , LABEL(0009-5012, 0009-5013, 0009-5014) | |