Drug Detail:Hepsera (Adefovir [ a-def-oh-vir ])
Drug Class: Nucleoside reverse transcriptase inhibitors (NRTIs)
Highlights of Prescribing Information
HEPSERA® (adefovir dipivoxil) Tablets, for oral use
Initial U.S. Approval: 2002
WARNING: SEVERE ACUTE EXACERBATIONS OF HEPATITIS, NEPHROTOXICITY, HIV RESISTANCE, LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS
See full prescribing information for complete boxed warning.
- Severe acute exacerbations of hepatitis may occur in patients who discontinue HEPSERA. Monitor hepatic function closely in these patients. (5.1)
- Chronic use of HEPSERA may result in nephrotoxicity in patients at risk of renal dysfunction or having underlying renal dysfunction. Monitor renal function closely in these patients. Dose adjustment may be required. (5.2)
- HIV resistance may emerge in chronic hepatitis B patients with unrecognized or untreated HIV infection. (5.3)
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues. (5.4)
Indications and Usage for Hepsera
HEPSERA is a nucleotide analogue indicated for the treatment of chronic hepatitis B in patients 12 years of age and older. (1)
Hepsera Dosage and Administration
- One tablet containing 10 mg adefovir dipivoxil once daily orally with or without food. (2.1)
- Dose adjustment in renal impairment for adults (2.2)
| Creatinine Clearance (mL/min)* | ||||
|---|---|---|---|---|
| Greater than or equal to 50 | 30–49 | 10–29 | Hemodialysis Patients | |
|
||||
| Recommended dose and dosing interval | 10 mg every 24 hours | 10 mg every 48 hours | 10 mg every 72 hours | 10 mg every 7 days following dialysis |
- No dose recommendations for (2.1):
- Non-hemodialysis patients with creatinine clearance less than 10 mL per minute.
- Adolescent patients with renal impairment.
Dosage Forms and Strengths
Tablets: 10 mg (3)
Contraindications
HEPSERA is contraindicated in patients with previously demonstrated hypersensitivity to any of the components of the product. (4)
Warnings and Precautions
- Severe acute exacerbations of hepatitis: Monitor hepatic function closely at repeated intervals for at least several months in patients who discontinue HEPSERA. (5.1)
- Nephrotoxicity: Monitor renal function during therapy for all patients, particularly those with pre-existing or other risks for renal impairment. Dose adjustment may be required. (5.2)
- HIV Resistance: Offer HIV testing to all patients prior to initiating HEPSERA. Untreated HIV may result in HIV resistance. (5.3)
- Lactic acidosis and severe hepatomegaly with steatosis: If suspected, suspend treatment. (5.4)
- Coadministration with Other Products: Do not administer HEPSERA concurrently with tenofovir-containing products. (5.5)
- Clinical Resistance: For patients with lamivudine-resistant HBV use adefovir dipivoxil in combination with lamivudine. For all patients, consider modifying treatment in case serum HBV DNA remains above 1000 copies/mL with continued treatment. (5.6)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence greater than 5%) in compensated liver disease patients were asthenia, headache, abdominal pain and nausea. (6.1) The most common adverse reaction in pre- and post-transplantation lamivudine-resistant liver disease patients was increased creatinine. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Gilead at (1-800-GILEAD-5) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Coadministration with drugs that reduce renal function or compete for active tubular secretion may increase serum concentrations of adefovir or the coadministered drug. Monitor for HEPSERA associated adverse events. (7)
Use In Specific Populations
- Pediatrics: Not recommended in children less than 12 years of age. (2.1, 8.4, 14.4)
- Renal Impairment: Dose adjustment may be required. (2.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2018
Full Prescribing Information
WARNING: SEVERE ACUTE EXACERBATIONS OF HEPATITIS, NEPHROTOXICITY, HIV RESISTANCE, LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS
Severe acute exacerbations of hepatitis have been reported in patients who have discontinued anti-Hepatitis B therapy including HEPSERA. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-Hepatitis B therapy. If appropriate, resumption of anti-Hepatitis B therapy may be warranted [See Warnings and Precautions (5.1)].
In patients at risk of or having underlying renal dysfunction, chronic administration of HEPSERA may result in nephrotoxicity. These patients should be monitored closely for renal function and may require dose adjustment [See Warnings and Precautions (5.2) and Dosage and Administration (2.2)].
HIV resistance may emerge in chronic hepatitis B patients with unrecognized or untreated Human Immunodeficiency Virus (HIV) infection treated with anti-hepatitis B therapies, such as therapy with HEPSERA, that may have activity against HIV [See Warnings and Precautions (5.3)].
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination with other antiretrovirals [See Warnings and Precautions (5.4)].
1. Indications and Usage for Hepsera
HEPSERA is indicated for the treatment of chronic hepatitis B in patients 12 years of age and older with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease.
This indication is based on histological, virological, biochemical, and serological responses in adult patients with HBeAg+ and HBeAg- chronic hepatitis B with compensated liver function, and with clinical evidence of lamivudine-resistant hepatitis B virus with either compensated or decompensated liver function.
For patients 12 to less than 18 years of age, the indication is based on virological and biochemical responses in patients with HBeAg+ chronic hepatitis B virus infection with compensated liver function.
2. Hepsera Dosage and Administration
2.1 Chronic Hepatitis B
The recommended dose of HEPSERA in chronic hepatitis B patients for patients 12 years of age and older with adequate renal function is 10 mg, once daily, taken orally, without regard to food. The optimal duration of treatment is unknown.
HEPSERA is not recommended for use in children less than 12 years of age.
2.2 Dose Adjustment in Renal Impairment
Significantly increased drug exposures were seen when HEPSERA was administered to adult patients with renal impairment [See Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. Therefore, the dosing interval of HEPSERA should be adjusted in adult patients with baseline creatinine clearance less than 50 mL per minute using the following suggested guidelines (See Table 1). The safety and effectiveness of these dosing interval adjustment guidelines have not been clinically evaluated.
Additionally, it is important to note that these guidelines were derived from data in patients with pre-existing renal impairment at baseline. They may not be appropriate for patients in whom renal insufficiency evolves during treatment with HEPSERA. Therefore, clinical response to treatment and renal function should be closely monitored in these patients.
| Creatinine Clearance (mL/min)* | Hemodialysis Patients | |||
|---|---|---|---|---|
| Greater than or equal to 50 | 30–49 | 10–29 | ||
|
||||
| Recommended dose and dosing interval | 10 mg every 24 hours | 10 mg every 48 hours | 10 mg every 72 hours | 10 mg every 7 days following dialysis |
The pharmacokinetics of adefovir have not been evaluated in non-hemodialysis patients with creatinine clearance less than 10 mL per minute; therefore, no dosing recommendation is available for these patients.
No clinical data are available to make dosing recommendations in adolescent patients with renal insufficiency [See Warnings and Precautions (5.2)].
3. Dosage Forms and Strengths
HEPSERA is available as tablets. Each tablet contains 10 mg of adefovir dipivoxil. The tablets are white and debossed with "10" and "GILEAD" on one side and the stylized figure of a liver on the other side.
4. Contraindications
HEPSERA is contraindicated in patients with previously demonstrated hypersensitivity to any of the components of the product.
5. Warnings and Precautions
5.1 Exacerbation of Hepatitis after Discontinuation of Treatment
Severe acute exacerbation of hepatitis has been reported in patients who have discontinued anti-hepatitis B therapy, including therapy with HEPSERA. Hepatic function should be monitored at repeated intervals with both clinical and laboratory follow-up for at least several months in patients who discontinue HEPSERA. If appropriate, resumption of anti-hepatitis B therapy may be warranted.
In clinical trials of HEPSERA, exacerbations of hepatitis (ALT elevations 10 times the upper limit of normal or greater) occurred in up to 25% of patients after discontinuation of HEPSERA. These events were identified in studies GS-98-437 and GS-98-438 (N=492). Most of these events occurred within 12 weeks of drug discontinuation. These exacerbations generally occurred in the absence of HBeAg seroconversion, and presented as serum ALT elevations in addition to re-emergence of viral replication. In the HBeAg-positive and HBeAg-negative studies in patients with compensated liver function, the exacerbations were not generally accompanied by hepatic decompensation. However, patients with advanced liver disease or cirrhosis may be at higher risk for hepatic decompensation. Although most events appear to have been self-limited or resolved with re-initiation of treatment, severe hepatitis exacerbations, including fatalities, have been reported. Therefore, patients should be closely monitored after stopping treatment.
5.2 Nephrotoxicity
Nephrotoxicity characterized by a delayed onset of gradual increases in serum creatinine and decreases in serum phosphorus was historically shown to be the treatment-limiting toxicity of adefovir dipivoxil therapy at substantially higher doses in HIV-infected patients (60 and 120 mg daily) and in chronic hepatitis B patients (30 mg daily). Chronic administration of HEPSERA (10 mg once daily) may result in delayed nephrotoxicity. The overall risk of nephrotoxicity in patients with adequate renal function is low. However, this is of special importance in patients at risk of or having underlying renal dysfunction and patients taking concomitant nephrotoxic agents such as cyclosporine, tacrolimus, aminoglycosides, vancomycin and non-steroidal anti-inflammatory drugs [See Adverse Reactions (6.2) and Clinical Pharmacology (12.3)]. It is recommended that creatinine clearance is calculated in all patients prior to initiating therapy with HEPSERA.
It is important to monitor renal function for all patients during treatment with HEPSERA, particularly for those with pre-existing or other risks for renal impairment. Patients with renal insufficiency at baseline or during treatment may require dose adjustment [See Dosage and Administration (2.2)]. The risks and benefits of HEPSERA treatment should be carefully evaluated prior to discontinuing HEPSERA in a patient with treatment-emergent nephrotoxicity.
5.3 HIV Resistance
Prior to initiating HEPSERA therapy, HIV antibody testing should be offered to all patients. Treatment with anti-hepatitis B therapies, such as HEPSERA, that have activity against HIV in a chronic hepatitis B patient with unrecognized or untreated HIV infection may result in emergence of HIV resistance. HEPSERA has not been shown to suppress HIV RNA in patients; however, there are limited data on the use of HEPSERA to treat patients with chronic hepatitis B co-infected with HIV.
5.4 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs alone or in combination with antiretrovirals.
A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogs to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with HEPSERA should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.5 Coadministration with Other Products
HEPSERA should not be used concurrently with products containing tenofovir disoproxil fumarate or tenofovir alafenamide.
5.6 Clinical Resistance
Resistance to adefovir dipivoxil can result in viral load rebound which may result in exacerbation of hepatitis B and, in the setting of diminished hepatic function, lead to liver decompensation and possible fatal outcome.
In order to reduce the risk of resistance in patients with lamivudine resistant HBV, adefovir dipivoxil should be used in combination with lamivudine and not as adefovir dipivoxil monotherapy.
In order to reduce the risk of resistance in all patients receiving adefovir dipivoxil monotherapy, a modification of treatment should be considered if serum HBV DNA remains above 1000 copies/mL with continued treatment.
Long-term (144 week) data from Study 438 (N=124) show that patients with HBV DNA levels greater than 1000 copies/mL at Week 48 of treatment with HEPSERA were at greater risk of developing resistance than patients with serum HBV DNA levels below 1000 copies/mL at Week 48 of therapy.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in other sections of the labeling:
- Severe acute exacerbations of Hepatitis [See Boxed Warning, Warnings and Precautions (5.1)]
- Nephrotoxicity [See Boxed Warning, Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical and laboratory evidence of exacerbations of hepatitis have occurred after discontinuation of treatment with HEPSERA.
Adverse reactions to HEPSERA identified from placebo-controlled and open label studies include the following: asthenia, headache, abdominal pain, diarrhea, nausea, dyspepsia, flatulence, increased creatinine, and hypophosphatemia.
The incidence of these adverse reactions in studies 437 and 438, where 522 patients with chronic hepatitis B and compensated liver disease received double-blind treatment with HEPSERA (N=294) or placebo (N=228) for 48 weeks is presented in Table 2. Patients who received open-label HEPSERA for up to 240 weeks in Study 438 reported adverse reactions similar in nature and severity to those reported in the first 48 weeks.
| Adverse Reaction | HEPSERA 10 mg (N=294) | Placebo (N=228) |
|---|---|---|
|
||
| Asthenia | 13% | 14% |
| Headache | 9% | 10% |
| Abdominal Pain | 9% | 11% |
| Nausea | 5% | 8% |
| Flatulence | 4% | 4% |
| Diarrhea | 3% | 4% |
| Dyspepsia | 3% | 2% |
No patients treated with HEPSERA developed a confirmed serum creatinine increase greater than or equal to 0.5 mg/dL from baseline or a confirmed phosphorus decrease to 2 mg/dL or less by Week 48. By Week 96, 2% of HEPSERA-treated patients, by Kaplan-Meier estimate, had increases in serum creatinine greater than or equal to 0.5 mg/dL from baseline (no placebo-controlled results were available for comparison beyond Week 48). For patients who chose to continue HEPSERA for up to 240 weeks in Study 438, 4 of 125 patients (3%) had a confirmed increase of 0.5 mg/dL from baseline. The creatinine elevation resolved in 1 patient who permanently discontinued treatment and remained stable in 3 patients who continued treatment. For 65 patients who chose to continue HEPSERA for up to 240 weeks in Study 437, 6 had a confirmed increase in serum creatinine of greater than or equal to 0.5 mg/dL from baseline with 2 patients discontinuing from the study due to the elevated serum creatinine concentration. See Adverse Reactions (6.2) for changes in serum creatinine in patients with underlying renal insufficiency at baseline.
6.3 Pediatric Patients
Assessment of adverse reactions is based on a placebo-controlled study (Study 518) in which 173 pediatric patients aged 2 to less than 18 years with chronic hepatitis B and compensated liver disease received double-blind treatment with HEPSERA (N=115), or placebo (N=58) for 48 weeks [See Clinical Studies (14.4) and Use In Specific Populations (8.4)].
The safety profile of HEPSERA in patients 12 to less than 18 years of age (N=56) was similar to that observed in adults. No pediatric patients treated with HEPSERA developed a confirmed serum creatinine increase greater than or equal to 0.5 mg/dL from baseline or a confirmed phosphorus decrease to less than 2 mg/dL by Week 48.
6.4 Post-Marketing Experience
In addition to adverse reaction reports from clinical trials, the following possible adverse reactions have also been identified during post-approval use of adefovir dipivoxil. Because these events have been reported voluntarily from a population of unknown size, estimates of frequency cannot be made.
Metabolism and Nutrition Disorders: hypophosphatemia
Gastrointestinal Disorders: pancreatitis
Musculoskeletal System and Connective Tissue Disorders: myopathy, osteomalacia (manifested as bone pain and may contribute to fractures), both associated with proximal renal tubulopathy
Renal and Urinary Disorders: renal failure, Fanconi syndrome, proximal renal tubulopathy
7. Drug Interactions
Since adefovir is eliminated by the kidney, coadministration of HEPSERA with drugs that reduce renal function or compete for active tubular secretion may increase serum concentrations of either adefovir and/or these coadministered drugs [See Clinical Pharmacology (12.3)].
Patients should be monitored closely for adverse events when HEPSERA is coadministered with drugs that are excreted renally or with other drugs known to affect renal function [See Warnings and Precautions (5.2)].
HEPSERA should not be administered in combination with products containing tenofovir disoproxil fumarate or tenofovir alafenamide [See Warnings and Precautions (5.5)].
8. Use In Specific Populations
8.5 Geriatric Use
Clinical studies of HEPSERA did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In general, caution should be exercised when prescribing to elderly patients since they have greater frequency of decreased renal or cardiac function due to concomitant disease or other drug therapy.
8.6 Patients with Impaired Renal Function
It is recommended that the dosing interval for HEPSERA be modified in adult patients with baseline creatinine clearance less than 50 mL per minute. The pharmacokinetics of adefovir have not been evaluated in non-hemodialysis patients with creatinine clearance less than 10 mL per minute or in adolescent patients with renal insufficiency; therefore, no dosing recommendations are available for these patients [See Dosage and Administration (2.2) and Warnings and Precautions (5.2)].
10. Overdosage
Doses of adefovir dipivoxil 500 mg daily for 2 weeks and 250 mg daily for 12 weeks have been associated with gastrointestinal side effects. If overdose occurs the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary.
Following a 10 mg single dose of HEPSERA, a four-hour hemodialysis session removed approximately 35% of the adefovir dose.
11. Hepsera Description
HEPSERA is the tradename for adefovir dipivoxil, a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV).
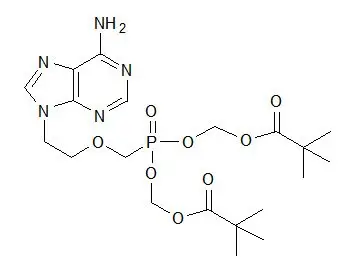
The chemical name of adefovir dipivoxil is 9-[2-[[bis[(pivaloyloxy)methoxy]-phosphinyl]-methoxy]ethyl]adenine. It has a molecular formula of C20H32N5O8P, a molecular weight of 501.48 and the following structural formula:
Adefovir dipivoxil is a white to off-white crystalline powder with an aqueous solubility of 19 mg/mL at pH 2.0 and 0.4 mg/mL at pH 7.2. It has an octanol/aqueous phosphate buffer (pH 7) partition coefficient (log p) of 1.91.
HEPSERA tablets are for oral administration. Each tablet contains 10 mg of adefovir dipivoxil and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, pregelatinized starch, and talc.
12. Hepsera - Clinical Pharmacology
12.3 Pharmacokinetics
Special Populations
Renal Impairment
In adults with moderately or severely impaired renal function or with end-stage renal disease (ESRD) requiring hemodialysis, Cmax, AUC, and half-life (T1/2) were increased compared to adults with normal renal function. It is recommended that the dosing interval of HEPSERA be modified in these patients [See Dosage and Administration (2.2)].
The pharmacokinetics of adefovir in non-chronic hepatitis B patients with varying degrees of renal impairment are described in Table 3. In this study, subjects received a 10 mg single dose of HEPSERA.
| Renal Function Group | Unimpaired | Mild | Moderate | Severe |
|---|---|---|---|---|
| Baseline creatinine clearance (mL/min) | >80 | 50–80 | 30–49 | 10–29 |
| (N=7) | (N=8) | (N=7) | (N=10) | |
| Cmax (ng/mL) | 17.8 ± 3.22 | 22.4 ± 4.04 | 28.5 ± 8.57 | 51.6 ± 10.3 |
| AUC 0–∞ (ng∙h/mL) | 201 ± 40.8 | 266 ± 55.7 | 455 ± 176 | 1240 ± 629 |
| CL/F (mL/min) | 469 ± 99.0 | 356 ± 85.6 | 237 ± 118 | 91.7 ± 51.3 |
| CLrenal (mL/min) | 231 ± 48.9 | 148 ± 39.3 | 83.9 ± 27.5 | 37.0 ± 18.4 |
A four-hour period of hemodialysis removed approximately 35% of the adefovir dose. The effect of peritoneal dialysis on adefovir removal has not been evaluated.
The pharmacokinetics of adefovir have not been studied in adolescent patients with renal dysfunction [See Use in Specific Populations (8.4)].
12.4 Microbiology
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term oral carcinogenicity studies of adefovir dipivoxil in mice and rats were carried out at exposures up to approximately 10 times (mice) and 4 times (rats) those observed in humans at the therapeutic dose for HBV infection. In both mouse and rat studies, adefovir dipivoxil was negative for carcinogenic findings. Adefovir dipivoxil was mutagenic in the in vitro mouse lymphoma cell assay (with or without metabolic activation). Adefovir induced chromosomal aberrations in the in vitro human peripheral blood lymphocyte assay without metabolic activation. Adefovir dipivoxil was not clastogenic in the in vivo mouse micronucleus assay and adefovir was not mutagenic in the Ames bacterial reverse mutation assay using S. typhimurium and E. coli strains in the presence or absence of metabolic activation. In reproductive toxicology studies, no evidence of impaired fertility was seen in male or female rats at systemic exposure approximately 19 times that achieved in humans at the therapeutic dose.
13.2 Animal Toxicology and/or Pharmacology
Renal tubular nephropathy characterized by histological alterations and/or increases in BUN and serum creatinine was the primary dose-limiting toxicity associated with administration of adefovir dipivoxil in animals. Nephrotoxicity was observed in animals at systemic exposures approximately 3–10 times higher than those in humans at the recommended therapeutic dose of 10 mg/day.
14. Clinical Studies
14.1 Studies 437 and 438 (Pivotal Studies)
HBeAg-Negative (Anti-HBe Positive/HBV DNA Positive) Chronic Hepatitis B
Study 438 was a randomized, double-blind, placebo-controlled study in patients who were HBeAg-negative at screening, and anti-HBe positive. The median age of patients was 46 years. Eighty-three percent were male, 66% were Caucasian, 30% were Asian and 41% had prior interferon-α treatment. At baseline, the median total Knodell HAI score was 10, the median serum HBV DNA level as measured by the Roche Amplicor Monitor PCR assay (LLOQ = 1000 copies/mL) was 7.08 log10 copies/mL, and the median ALT was 2.3 times the upper limit of normal.
The primary efficacy endpoint in both studies was histological improvement at Week 48; results of which are shown in Table 4.
| Study 437 | Study 438 | |||
|---|---|---|---|---|
| HEPSERA 10 mg (N=168) | Placebo (N=161) | HEPSERA 10 mg (N=121) | Placebo (N=57) |
|
|
||||
| Improvement† | 53% | 25% | 64% | 35% |
| No Improvement | 37% | 67% | 29% | 63% |
| Missing/Unassessable Data | 10% | 7% | 7% | 2% |
Table 5 illustrates the changes in Ishak Fibrosis Score by treatment group.
| Study 437 | Study 438 | |||
|---|---|---|---|---|
| Number of Adequate Biopsy Pairs | HEPSERA 10 mg (N=152) | Placebo (N=149) | HEPSERA 10 mg (N=113) | Placebo (N=56) |
|
||||
| Ishak Fibrosis Score | ||||
| Improved* | 34% | 19% | 34% | 14% |
| Unchanged | 55% | 60% | 62% | 50% |
| Worsened* | 11% | 21% | 4% | 36% |
At Week 48, improvement was seen with respect to mean change in serum HBV DNA (log10 copies/mL), normalization of ALT, and HBeAg seroconversion as compared to placebo in patients receiving HEPSERA (Table 6).
| Study 437 | Study 438 | |||
|---|---|---|---|---|
| HEPSERA 10 mg (N=171) | Placebo (N=167) | HEPSERA 10 mg (N=123) | Placebo (N=61) |
|
|
||||
| Mean change ± SD in serum HBV DNA from baseline (log10 copies/mL) | –3.57 ± 1.64 | –0.98 ± 1.32 | –3.65 ± 1.14 | –1.32 ± 1.25 |
| ALT normalization | 48% | 16% | 72% | 29% |
| HBeAg seroconversion | 12% | 6% | NA* | NA* |
14.2 Study 435 (Pre- and Post-Liver Transplantation Patients)
HEPSERA was also evaluated in an open-label, uncontrolled study of 467 chronic hepatitis B patients pre- (N=226) and post- (N=241) liver transplantation with clinical evidence of lamivudine-resistant hepatitis B virus (Study 435). At baseline, 60% of pre-liver transplantation patients were classified as Child-Pugh-Turcotte score of Class B or C. The median baseline HBV DNA as measured by the Roche Amplicor Monitor PCR assay (LLOQ = 1000 copies/mL) was 7.4 and 8.2 log10 copies/mL, and the median baseline ALT was 1.8 and 2.0 times the upper limit of normal in pre- and post-liver transplantation patients, respectively. Results of this study are displayed in Table 7. Treatment with HEPSERA resulted in a similar reduction in serum HBV DNA regardless of the patterns of lamivudine-resistant HBV DNA polymerase mutations at baseline. The significance of the efficacy results listed in Table 7 as they relate to clinical outcomes is not known.
| Efficacy Parameter* | Pre-Liver Transplantation (N=226) | Post-Liver Transplantation (N=241) |
|---|---|---|
|
||
| Mean change ± SD in HBV DNA from baseline (log10 copies/mL) | –3.7 ± 1.6 (N=117) | –4.0 ± 1.6 (N=164) |
| Proportion with undetectable HBV DNA (< 1000 copies/mL)† | 77/109 (71%) | 64/159 (40%) |
| Stable or improved Child-Pugh-Turcotte score | 86/90 (96%) | 107/115 (93%) |
| Normalization of:‡ | ||
| ALT | 61/82 (74%) | 56/110 (51%) |
| Albumin | 43/54 (80%) | 21/26 (81%) |
| Bilirubin | 38/68 (58%) | 29/38 (76%) |
| Prothrombin time | 39/46 (85%) | 5/9 (56%) |
14.3 Study 461 (Clinical Evidence of Lamivudine Resistance)
In Study 461, a double-blind, active controlled study in 59 chronic hepatitis B patients with clinical evidence of lamivudine-resistant hepatitis B virus, patients were randomized to receive either HEPSERA monotherapy or HEPSERA in combination with lamivudine 100 mg or lamivudine 100 mg alone. At Week 48, the mean ± SD decrease in serum HBV DNA as measured by the Roche Amplicor Monitor PCR assay (LLOQ = 1000 copies/mL) was 4.00 ± 1.41 log10 copies/mL for patients treated with HEPSERA and 3.46 ± 1.10 log10 copies/mL for patients treated with HEPSERA in combination with lamivudine. There was a mean decrease in serum HBV DNA of 0.31 ± 0.93 log10 copies/mL in patients receiving lamivudine alone. ALT normalized in 47% of patients treated with HEPSERA, in 53% of patients treated with HEPSERA in combination with lamivudine, and 5% of patients treated with lamivudine alone. The significance of these findings as they relate to clinical outcomes is not known.
14.4 Study 518 (Pediatric Study)
Study 518 was a double-blind, placebo-controlled, study in which 173 pediatric patients (ages 2 to less than 18 years) with chronic hepatitis B (CHB) infection and elevated ALT were randomized 2:1 (115 receiving adefovir dipivoxil and 58 receiving placebo). Randomization was stratified by prior treatment and age 2 to less than 7 years old (cohort 1), 7 to less than 12 years old (cohort 2), and 12 to less than 18 years old (cohort 3). All patients in cohort 3 received 10 mg tablet formulation; all patients in cohorts 1 and 2 received an investigational suspension formulation (0.3 mg/kg/day cohort 1, 0.25 mg/kg/day cohort 2) once daily. The primary efficacy endpoint was HBV DNA less than 1000 copies/mL plus normalization of ALT at the end of Week 48.
In cohort 3 (N=83), significantly more patients treated with HEPSERA achieved the primary efficacy endpoint at the end of 48 weeks of blinded treatment (23%) when compared to placebo-treated patients (0%). The proportion of patients from cohorts 1 and 2 who responded to treatment with adefovir dipivoxil was not statistically significant when compared to the placebo arm, although the adefovir plasma concentrations in these patients were comparable to those observed in older patients. Overall, 22 of 115 (19%) of pediatric patients who received adefovir dipivoxil versus 1 of 58 (2%) of placebo treated patients responded to treatment by Week 48 [See Adverse Reactions (6.3), Use In Specific Populations (8.4) and Clinical Pharmacology (12.3, 12.4)].
16. How is Hepsera supplied
HEPSERA is available as tablets. Each tablet contains 10 mg of adefovir dipivoxil. The tablets are white and debossed with "10" and "GILEAD" on one side and the stylized figure of a liver on the other side. They are packaged as follows: Bottles of 30 tablets (NDC 61958-0501-1) containing desiccant (silica gel) and closed with a child-resistant closure.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Inform patients of the potential risks and benefits of HEPSERA and of alternative modes of therapy.
- Instruct patients to:
- —
- Follow a regular dosing schedule to avoid missing doses.
- —
- Immediately report any severe abdominal pain, muscle pain, yellowing of the eyes, dark urine, pale stools, and/or loss in appetite.
- —
- Inform their doctor or pharmacist if they develop any unusual symptom(s), or if any known symptom persists or worsens.
- Advise patients that:
- —
- The optimal duration of HEPSERA treatment and the relationship between treatment response and long-term outcomes such as hepatocellular carcinoma or decompensated cirrhosis are not known.
- —
- Patients should not discontinue HEPSERA without first informing their physician [See Warnings and Precautions (5.1)].
- —
- Routine laboratory monitoring and follow-up with a physician is important during HEPSERA therapy.
- —
- Obtaining HIV antibody testing prior to starting HEPSERA is important [See Warnings and Precautions (5.3)].
- —
- HEPSERA should not be administered concurrently with products containing tenofovir disoproxil fumarate or tenofovir alafenamide [See Warnings and Precautions (5.5)].
- —
- Lamivudine-resistant patients should use HEPSERA in combination with lamivudine and not as HEPSERA monotherapy [See Warnings and Precautions. (5.6)].
- Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant women exposed to HEPSERA [see Use in Specific Populations (8.1)].
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: December 2018 | ||
| PATIENT INFORMATION
HEPSERA (hep-SER-rah) (adefovir dipivoxil) tablets |
|||
| Read this Patient Information before you start taking HEPSERA and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. | |||
| What is the most important information I should know about HEPSERA?
HEPSERA can cause serious side effects, including: |
|||
|
|||
|
|
||
|
|||
|
|
||
| You may be more likely to get lactic acidosis or serious liver problems if you are female, are very overweight (obese), or have been taking nucleoside analog medicines for a long time. | |||
| What is HEPSERA?
HEPSERA is a medicine used to treat people 12 years of age and older with chronic (long-lasting) infections with active hepatitis B virus. HEPSERA is not for use in children under 12 years of age.
|
|||
| Who should not take HEPSERA?
Do not take HEPSERA if you are allergic to any of the ingredients in HEPSERA. See the end of this leaflet for a complete list of the ingredients in HEPSERA. |
|||
| What should I tell my healthcare provide before taking HEPSERA? Before taking HEPSERA, tell your doctor about all of your medical conditions, including if you:
Especially tell your doctor if you take a medicine that contains tenofovir disoproxil fumarate or tenofovir alafenamide. |
|||
How should I take HEPSERA?
|
|||
| What are the possible side effects of HEPSERA?
HEPSERA can cause serious side effects. See "What is the most important information I should know about HEPSERA?" The most common side effects of HEPSERA are weakness, headache, stomach pain, and nausea. These are not all the possible side effects of HEPSERA. For more information, ask your doctor or pharmacist. Call your doctor for advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 |
|||
How should I store HEPSERA?
|
|||
| General information about the safe and effective use of HEPSERA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use HEPSERA for a condition for which it was not prescribed. Do not give HEPSERA to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about HEPSERA that is written for health professionals. |
|||
| What are the Ingredients in HEPSERA?
Active Ingredient: adefovir dipivoxil Inactive Ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, pregelatinized starch, and talc Manufactured for: Gilead Sciences, Inc. Foster City, CA 94404 HEPSERA is a registered trademark of Gilead Sciences, Inc., or its related companies. ©2018 Gilead Sciences, Inc. 21449-GS-014 For more information, go to www.Gilead.com or call 1-800-445-3235. |
|||
| HEPSERA
adefovir dipivoxil tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Gilead Sciences, Inc. (185049848) |