Drug Detail:Hyftor (Sirolimus)
Drug Class: MTOR inhibitors Selective immunosuppressants
Highlights of Prescribing Information
HYFTOR™ (sirolimus topical gel)
Initial U.S. Approval: 1999
Indications and Usage for Hyftor
HYFTOR is an mTOR inhibitor immunosuppressant indicated for the treatment of facial angiofibroma associated with tuberous sclerosis in adults and pediatric patients 6 years of age and older. ( 1)
Hyftor Dosage and Administration
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to HYFTOR initiation. ( 2)
- Apply to the skin of the face affected with angiofibroma twice daily. ( 2)
- The maximum daily dosage is:
- 600 mg (2 cm) for patients 6 to 11 years of age. ( 2)
- 800 mg (2.5 cm) for patients 12 years of age and older. ( 2)
- Do not use with occlusive dressings. ( 2)
- For topical use only. Not for oral, ophthalmic, or intravaginal use. ( 2)
Dosage Forms and Strengths
Topical gel, 0.2%: 2 mg of sirolimus per gram. ( 3)
Contraindications
History of hypersensitivity to sirolimus or any other component of HYFTOR. ( 4)
Warnings and Precautions
- Hypersensitivity Reactions: Oral sirolimus has been associated with hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis, and hypersensitivity vasculitis. Discontinue HYFTOR immediately if symptoms of hypersensitivity occur. ( 5.1)
- Serious Infection: Serious infections, including opportunistic infections and latent viral infections, such as progressive multifocal leukoencephalopathy, have been reported with oral sirolimus. Discontinue HYFTOR immediately if symptoms of infection occur. ( 5.2)
- Malignancy: Oral sirolimus has been associated with malignancy, including lymphoma and skin cancer. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using HYFTOR. ( 5.3)
- Hyperlipidemia: Oral sirolimus has been associated with increased serum cholesterol and triglycerides requiring treatment. Monitor for hyperlipidemia during treatment. ( 5.4)
- Interstitial Lung Disease (ILD)/Non-infectious Pneumonitis: Oral sirolimus has been associated with ILD, sometimes fatal. Discontinue HYFTOR if ILD symptoms occur. ( 5.5)
- Immunizations: During treatment with HYFTOR, vaccinations may be less effective. Avoid use of live vaccines during treatment with HYFTOR. ( 5.6)
- Embryo-Fetal Toxicity: Based on animal studies, HYFTOR can cause fetal harm. Use of effective contraception is recommended for females of reproductive potential prior to and throughout treatment, and for 12 weeks after final dose of HYFTOR. ( 5.7, 8.1, 8.3)
- Male Infertility: Oral sirolimus has been associated with azoospermia and oligospermia. Advise males that HYFTOR may impair fertility. ( 5.8, 8.3, 13.1)
Adverse Reactions/Side Effects
Most common adverse reactions (≥1%) are dry skin, application site irritation, pruritus, acne, acneiform dermatitis, ocular hyperemia, skin hemorrhage, and skin irritation. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Nobelpharma America, LLC at 1 (877) 375-0825 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 Inhibitors: During concomitant use of HYFTOR with CYP3A4 inhibitors, monitor for adverse reactions of HYFTOR. ( 7.1)
- Substrates and Inhibitors of CYP3A: During concomitant use of HYFTOR with drugs that are both substrates and inhibitors of CYP3A, monitor for adverse reactions of the CYP3A substrate and inhibitor. ( 7.2)
Use In Specific Populations
Lactation: Breastfeeding not recommended. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2022
Full Prescribing Information
1. Indications and Usage for Hyftor
HYFTOR is indicated for the treatment of facial angiofibroma associated with tuberous sclerosis in adults and pediatric patients 6 years of age and older.
2. Hyftor Dosage and Administration
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to HYFTOR initiation [see Warning and Precautions (5.6)] .
- Apply HYFTOR to the skin of the face affected with angiofibroma twice daily in the morning and at bedtime.
- The maximum recommended daily dosage is:
- 600 mg (2 cm) for pediatric patients 6 to 11 years of age
- 800 mg (2.5 cm) for adults and pediatric patients 12 years of age and older
- If symptoms do not improve within 12 weeks of treatment, reevaluate the need for continuing HYFTOR.
- Do not use HYFTOR with occlusive dressings.
- For topical use only. Not for oral, ophthalmic, or intravaginal use.
3. Dosage Forms and Strengths
Topical gel , 0.2%: Each gram contains 2 mg of sirolimus in a colorless and transparent gel in 10-gram tubes.
4. Contraindications
HYFTOR is contraindicated in patients with a history of hypersensitivity to sirolimus or any other component of HYFTOR. Reactions to sirolimus have included anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis, and hypersensitivity vasculitis [see Warning and Precautions (5.1)] .
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis, and hypersensitivity vasculitis have been associated with the oral administration of sirolimus. The concomitant use of HYFTOR with other drugs known to cause angioedema, such as angiotensin-converting enzyme (ACE) inhibitors, may increase the risk of developing angioedema. Elevated sirolimus levels (with or without concomitant ACE inhibitors) may also potentiate angioedema. Discontinue HYFTOR immediately if symptoms of hypersensitivity occur.
5.2 Serious Infection
Serious infections, including opportunistic infections, have been reported after oral administration of sirolimus. Cases of progressive multifocal leukoencephalopathy (PML), sometimes fatal have been reported in patients treated with oral sirolimus. Discontinue HYFTOR immediately if symptoms of infection occur.
5.3 Malignancy
Lymphoma and other malignancies, particularly of the skin, have been observed after oral administration of sirolimus. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using HYFTOR. If patients need to be outdoors while using HYFTOR, they should wear protective clothing and discuss other sun protection measures with their physician.
5.4 Hyperlipidemia
Increased serum cholesterol and triglycerides requiring treatment have been observed with oral administration of sirolimus. Monitor for hyperlipidemia during treatment with HYFTOR.
5.5 Interstitial Lung Disease/Non-Infectious Pneumonitis
Cases of interstitial lung disease [ILD] (including pneumonitis, bronchiolitis obliterans organizing pneumonia [BOOP], and pulmonary fibrosis), some fatal, with no identified infectious etiology have occurred in patients receiving oral sirolimus. In some cases, the ILD has resolved upon discontinuation or dosage reduction of oral sirolimus. Discontinue HYFTOR immediately if symptoms of ILD occur.
5.6 Immunizations
During treatment with HYFTOR, vaccinations may be less effective. Complete all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating treatment with HYFTOR. The use of live vaccines should be avoided during treatment with HYFTOR.
5.7 Embryo-Fetal Toxicity
Based on animal studies and the mechanism of action, oral sirolimus can cause fetal harm when administered to a pregnant woman. In animal studies, oral sirolimus caused embryo-fetal toxicity when administered during the period of organogenesis at maternal exposures that were equal to or less than human exposures at the recommended lowest starting dose. HYFTOR is systemically absorbed after topical administration and may result in fetal exposure. Advise pregnant women of the potential risk to a fetus. Advise female patients of reproductive potential to avoid becoming pregnant. They should use effective contraception prior to, throughout treatment and for 12 weeks after the final dose of HYFTOR [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1, 12.3)].
5.8 Male Infertility
Azoospermia or oligospermia has been observed after oral administration of sirolimus [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)]. Sirolimus is an anti-proliferative drug and affects rapidly dividing cells like the germ cells. Advise males that HYFTOR may impair fertility.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a randomized, double-blind, vehicle-controlled trial, subjects aged 6 years and older with facial angiofibroma associated with tuberous sclerosis applied HYFTOR twice daily for 12 weeks. A total of 30 subjects were treated with HYFTOR and 32 with the vehicle. The majority of the subjects were female (54.8%). A total of 40.3% were less than 18 years of age.
The most common adverse reactions reported by ≥1% of subjects treated with HYFTOR and more frequently than in subjects treated with vehicle are presented in Table 1. Adverse reactions occurred with similar frequency in pediatric subjects 6 years of age and older.
| Preferred Term | HYFTOR
N = 30 | Vehicle
N = 32 |
|---|---|---|
|
||
| Dry skin * | 12 (40%) | 4 (13%) |
| Application site irritation | 11 (37%) | 9 (28%) |
| Pruritus | 5 (17%) | 4 (13%) |
| Acne | 2 (7%) | 0 (0%) |
| Acneiform dermatitis | 1 (3%) | 0 (0%) |
| Ocular hyperemia | 1 (3%) | 0 (0%) |
| Skin hemorrhage | 1 (3%) | 0 (0%) |
| Skin irritation | 1 (3%) | 0 (0%) |
In a 104-week, open-label safety trial, the most common adverse reactions associated with HYFTOR application were application site irritation (31%), dry skin (28%), acne (20%), pruritus (9%), eye irritation (9%), erythema (7%), acneiform dermatitis (6%), contact dermatitis (5%), solar dermatitis (1%), and photosensitivity reaction (1%). Adverse reactions occurred with similar frequency in adult and pediatric subjects 6 years of age and older.
7. Drug Interactions
7.1 Effects of Other Drugs on HYFTOR
Table 2 presents clinically significant drug interactions involving drugs that affect HYFTOR.
| Clinical Impact | Concomitant use of HYFTOR with inhibitors of CYP3A4 has the potential to increase the systemic exposure of sirolimus and increase the risk of HYFTOR adverse reactions. |
| Intervention | Monitor for adverse reactions of HYFTOR. |
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of HYFTOR have been established in pediatric patients aged 6 years and older for the topical treatment of facial angiofibroma associated with tuberous sclerosis. Use of HYFTOR in this age group is supported by data from a randomized, vehicle-controlled, double-blind 12-week trial along with additional efficacy and long-term safety data from a 104-week open label safety trial. A total of 13 pediatric subjects aged 6 years to 17 years received HYFTOR in the Phase 3 clinical trial along with 48 pediatric subjects aged 3 years to 17 years in the 104-week open label safety trial. Adverse reactions occurred with similar frequency in adult and pediatric subjects [see Adverse Reaction (6.1), Clinical Studies (14)].
The safety and effectiveness of HYFTOR for this indication have not been established in pediatric patients less than 6 years of age.
8.5 Geriatric Use
Clinical studies of HYFTOR did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
11. Hyftor Description
HYFTOR™ (sirolimus topical gel) 0.2% is an mTOR inhibitor immunosuppressant for topical use. Each gram contains 2 mg of sirolimus, which is solubilized in a gel consisting of alcohol 51%, Carbomer 940, purified water, and trolamine.
Chemically, sirolimus is designated as (3 S,6 R,7 E,9 R,10 R,12 R,14 S,15 E,17 E,19 E,21 S,23 S,26 R,27 R,34a S)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34,34a-hexadecahydro-9,27-dihydroxy-3-[(1 R)-2-[(1 S,3 R,4 R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3 H-pyrido[2,1- c][1,4]oxaazacyclohentriacontine-1,5,11,28,29(4 H,6 H,31 H)-pentone. It has the following structural formula:
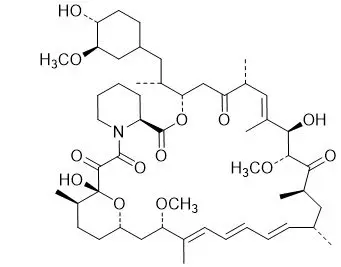
Sirolimus is a white to off-white powder and is insoluble in water, but freely soluble in chloroform, acetone and acetonitrile. Sirolimus has a molecular formula of C 51H 79NO 13 and a molecular weight of 914.19.
12. Hyftor - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of sirolimus in the treatment of angiofibroma associated with tuberous sclerosis is unknown. Tuberous sclerosis is associated with genetic defects in TSC1 and TSC2 which leads to the constitutive activation of mammalian target of rapamycin (mTOR). Sirolimus inhibits mTOR activation.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oral carcinogenicity studies were conducted in mice and rats. In an oral carcinogenicity study conducted in female mice, sirolimus administered once daily for 86 weeks was associated with a statistically significant increase in malignant lymphoma at all dose levels compared with control animals. In a second oral mouse carcinogenicity study, sirolimus-related hepatocellular adenoma and carcinoma were induced in male mice. In an oral carcinogenicity study conducted in rats with sirolimus there were no significant findings.
Sirolimus was not genotoxic in the in vitro bacterial reverse mutation assay, the Chinese hamster ovary cell chromosomal aberration assay, the mouse lymphoma cell forward mutation assay, or the in vivo mouse micronucleus assay.
Fertility was decreased in both male and female rats following oral administration of sirolimus 2.0 and 0.5 mg/kg, respectively. In male rats, atrophy of testes, epididymides, prostate, seminiferous tubules and/or reduced sperm counts were observed. In female rats, decreased ovarian and uterine weights and decreased implantation were observed. Testicular tubular degeneration was also seen in a 4-week intravenous study of sirolimus in monkeys at 0.1 mg/kg.
14. Clinical Studies
A single, randomized, double-blind, vehicle-controlled, multi-center, Phase 3 trial was conducted in Japan to evaluate HYFTOR for the treatment of adults and pediatric patients 6 years of age and older with facial angiofibroma associated with tuberous sclerosis (NCT02635789). A total of 62 Japanese subjects with 3 or more angiofibromas (≥2 mm in diameter with redness in each) on the face were enrolled in this trial. Overall, 28 subjects (45%) were male and 34 (55%) were female. A total of 25 subjects (40%) were between 6 and <18 years of age. In this trial, subjects applied either HYFTOR or vehicle twice daily to the skin of their face affected with angiofibroma for 12 weeks.
The efficacy was assessed by the investigator (live assessment) based on the composite improvement from baseline in size and redness of facial angiofibroma, using subjects' baseline photographs as reference. The proportion of subjects assessed as 'Improved' or 'Markedly Improved' at Week 12 is presented in Table 3. An assessment of 'Improved' was defined as at least a 50% reduction in the size and a 2-level reduction in redness and an assessment of 'Markedly Improved' was defined as at least a 75% reduction in the size and a 3-level reduction in redness.
| Proportion of Subjects Assessed by the Investigator as: | HYFTOR
N=30 | Vehicle
N=32 |
|---|---|---|
| 'Improved' or 'Markedly Improved' | 23% | 6% |
| 'Improved' | 13% | 3% |
| 'Markedly Improved' | 10% | 3% |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Issued: 2/2022 | |||
| PATIENT INFORMATION
HYFTOR™ (hyfe tore) (sirolimus topical gel) |
||||
| Important: HYFTOR is for use on the skin only (topical use). Do not use HYFTOR in your mouth, eyes, or vagina. | ||||
| What is HYFTOR?
HYFTOR is a prescription medicine that is used on the skin (topical) to treat adults and children 6 years of age and older with a type of noncancerous tumor called angiofibroma on your face caused by the genetic condition tuberous sclerosis. It is not known if HYFTOR is safe and effective in children under 6 years of age. |
||||
| Do not use HYFTOR if you are allergic to sirolimus or any of the other ingredients in HYFTOR. See the end of this leaflet for a complete list of ingredients in HYFTOR. | ||||
Before using HYFTOR, tell your healthcare provider about all of your medical conditions, including if you:
|
||||
How should I use HYFTOR?
|
||||
| What should I avoid while using HYFTOR?
Limit your exposure to sunlight and ultraviolet light, such as tanning beds and ultraviolet light therapy, during treatment with HYFTOR. Wear clothing that covers your skin if you need to go outside. Talk with your healthcare provider about other ways you can protect your skin from the sun. |
||||
| What are the possible side effects of HYFTOR?
HYFTOR may cause serious side effects, including:
|
||||
|
|
|||
HYFTOR may cause fertility problems in males and females, which may affect your ability to have children. Talk to your healthcare provider if this is a concern for you. These are not all the possible side effects of HYFTOR. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
How should I store HYFTOR ?
|
||||
| General information about the safe and effective use of HYFTOR.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use HYFTOR for a condition for which it was not prescribed. Do not give HYFTOR to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about HYFTOR that is written for health professionals. |
||||
| What are the ingredients in HYFTOR?
Active ingredient: sirolimus Inactive ingredients: alcohol 51%, Carbomer 940, purified water, and trolamine. Distributed by: Nobelpharma America, LLC 4520 East-West Highway, Suite 400, Bethesda, MD 20814 For more information, go to www.Hyftor.com or call 887-375-0825. |
||||
| HYFTOR
sirolimus gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Nobelpharma America, LLC (117340493) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Clinical, Inc | 079209266 | analysis(73683-101) , label(73683-101) , pack(73683-101) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Toyo Pharmaceutical Co., Ltd. | 717792357 | manufacture(73683-101) , analysis(73683-101) | |





