Drug Detail:Inomax (inhalation gas) (Nitric oxide (inhalation gas) [ nye-trik-ox-ide ])
Drug Class: Miscellaneous respiratory agents
Highlights of Prescribing Information
INOMAX® (nitric oxide) gas, for inhalation
Initial U.S. Approval: 1999
Indications and Usage for Inomax
INOmax is a vasodilator indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents. (1)
Inomax Dosage and Administration
The recommended dose is 20 ppm, maintained for up to 14 days or until the underlying oxygen desaturation has resolved (2.1).
Doses greater than 20 ppm are not recommended (2.1, 5.2)
Administration:
- Avoid abrupt discontinuation (2.2, 5.1).
Dosage Forms and Strengths
INOmax (nitric oxide) is a gas available in 800 and 4,880 ppm concentrations (3).
Contraindications
Neonates dependent on right-to-left shunting of blood (4).
Warnings and Precautions
Rebound: Abrupt discontinuation of INOmax may lead to worsening oxygenation and increasing pulmonary artery pressure (5.1).
Methemoglobinemia: Methemoglobin increases with the dose of nitric oxide; following discontinuation or reduction of nitric oxide, methemoglobin levels return to baseline over a period of hours (5.2).
Elevated NO2 Levels: Monitor NO2 levels (5.3).
Heart Failure: In patients with pre-existing left ventricular dysfunction, INOmax may increase pulmonary capillary wedge pressure leading to pulmonary edema (5.4).
Adverse Reactions/Side Effects
The most common adverse reaction is hypotension. (6).
To report SUSPECTED ADVERSE REACTIONS, contact INO Therapeutics at 1-877-566-9466 and http://www.inomax.com/ or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Nitric oxide donor compounds may increase the risk of developing methemoglobinemia (7).
Revised: 4/2023
Related/similar drugs
nitric oxide, caffeine / sodium benzoateFull Prescribing Information
1. Indications and Usage for Inomax
INOmax® is indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents.
3. Dosage Forms and Strengths
INOmax (nitric oxide) gas is available in 800 and 4,880 ppm concentrations.
4. Contraindications
INOmax is contraindicated in neonates dependent on right-to-left shunting of blood.
5. Warnings and Precautions
5.1 Rebound Pulmonary Hypertension Syndrome following Abrupt Discontinuation
Wean from INOmax [see Dosage and Administration (2.2)]. Abrupt discontinuation of INOmax may lead to worsening oxygenation and increasing pulmonary artery pressure, i.e., Rebound Pulmonary Hypertension Syndrome. Signs and symptoms of Rebound Pulmonary Hypertension Syndrome include hypoxemia, systemic hypotension, bradycardia, and decreased cardiac output. If Rebound Pulmonary Hypertension occurs, reinstate INOmax therapy immediately.
5.2 Hypoxemia from Methemoglobinemia
Nitric oxide combines with hemoglobin to form methemoglobin, which does not transport oxygen. Methemoglobin levels increase with the dose of INOmax; it can take 8 hours or more before steady-state methemoglobin levels are attained. Monitor methemoglobin and adjust the dose of INOmax to optimize oxygenation.
If methemoglobin levels do not resolve with decrease in dose or discontinuation of INOmax, additional therapy may be warranted to treat methemoglobinemia [see Overdosage (10)].
5.3 Airway Injury from Nitrogen Dioxide
Nitrogen dioxide (NO2) forms in gas mixtures containing NO and O2. Nitrogen dioxide may cause airway inflammation and damage to lung tissues.
If there is an unexpected change in NO2 concentration, or if the NO2 concentration reaches 3 ppm when measured in the breathing circuit, then the delivery system should be assessed in accordance with the Nitric Oxide Delivery System O&M Manual troubleshooting section, and the NO2 analyzer should be recalibrated. The dose of INOmax and/or FiO2 should be adjusted as appropriate.
5.4 Worsening Heart Failure
Patients with left ventricular dysfunction treated with INOmax may experience pulmonary edema, increased pulmonary capillary wedge pressure, worsening of left ventricular dysfunction, systemic hypotension, bradycardia and cardiac arrest. Discontinue INOmax while providing symptomatic care.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed elsewhere in the label;
Hypoxemia [see Warnings and Precautions (5.2)]
Worsening Heart Failure [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from the clinical studies does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Controlled studies have included 325 patients on INOmax doses of 5 to 80 ppm and 251 patients on placebo. Total mortality in the pooled trials was 11% on placebo and 9% on INOmax, a result adequate to exclude INOmax mortality being more than 40% worse than placebo.
In both the NINOS and CINRGI studies, the duration of hospitalization was similar in INOmax and placebo-treated groups.
From all controlled studies, at least 6 months of follow-up is available for 278 patients who received INOmax and 212 patients who received placebo. Among these patients, there was no evidence of an adverse effect of treatment on the need for rehospitalization, special medical services, pulmonary disease, or neurological sequelae.
In the NINOS study, treatment groups were similar with respect to the incidence and severity of intracranial hemorrhage, Grade IV hemorrhage, periventricular leukomalacia, cerebral infarction, seizures requiring anticonvulsant therapy, pulmonary hemorrhage, or gastrointestinal hemorrhage.
In CINRGI, the only adverse reaction (>2% higher incidence on INOmax than on placebo) was hypotension (14% vs. 11%).
8. Use In Specific Populations
8.4 Pediatric Use
The safety and efficacy of nitric oxide for inhalation has been demonstrated in term and near-term neonates with hypoxic respiratory failure associated with evidence of pulmonary hypertension [see Clinical Studies (14.1)]. Additional studies conducted in premature neonates for the prevention of bronchopulmonary dysplasia have not demonstrated substantial evidence of efficacy [see Clinical Studies (14.3)]. No information about its effectiveness in other age populations is available.
10. Overdosage
Overdosage with INOmax is manifest by elevations in methemoglobin and pulmonary toxicities associated with inspired NO2. Elevated NO2 may cause acute lung injury. Elevations in methemoglobin reduce the oxygen delivery capacity of the circulation. In clinical studies, NO2 levels >3 ppm or methemoglobin levels >7% were treated by reducing the dose of, or discontinuing, INOmax.
Methemoglobinemia that does not resolve after reduction or discontinuation of therapy can be treated with intravenous vitamin C, intravenous methylene blue, or blood transfusion, based upon the clinical situation.
11. Inomax Description
INOmax (nitric oxide gas) is a drug administered by inhalation. Nitric oxide, the active substance in INOmax, is a pulmonary vasodilator. INOmax 800 ppm is a gaseous blend of nitric oxide (0.08%) and nitrogen (99.92%). INOmax 4,880™ ppm is a gaseous blend of nitric oxide (0.488%) and nitrogen (99.51%). INOmax 800 ppm is supplied in aluminum cylinders as a compressed gas under high pressure (2,000 pounds per square inch [psi]). INOmax 4,880 ppm is supplied in aluminum cylinders as a compressed gas under high pressure (3,000 psi).
The structural formula of nitric oxide (NO) is shown below:
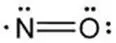
12. Inomax - Clinical Pharmacology
12.1 Mechanism of Action
Nitric oxide relaxes vascular smooth muscle by binding to the heme moiety of cytosolic guanylate cyclase, activating guanylate cyclase and increasing intracellular levels of cyclic guanosine 3',5'-monophosphate, which then leads to vasodilation. When inhaled, nitric oxide selectively dilates the pulmonary vasculature, and because of efficient scavenging by hemoglobin, has minimal effect on the systemic vasculature.
INOmax appears to increase the partial pressure of arterial oxygen (PaO2) by dilating pulmonary vessels in better ventilated areas of the lung, redistributing pulmonary blood flow away from lung regions with low ventilation/perfusion (V/Q) ratios toward regions with normal ratios.
Absorption and Distribution
Nitric oxide is absorbed systemically after inhalation. Most of it traverses the pulmonary capillary bed where it combines with hemoglobin that is 60% to 100% oxygen-saturated. At this level of oxygen saturation, nitric oxide combines predominantly with oxyhemoglobin to produce methemoglobin and nitrate. At low oxygen saturation, nitric oxide can combine with deoxyhemoglobin to transiently form nitrosylhemoglobin, which is converted to nitrogen oxides and methemoglobin upon exposure to oxygen. Within the pulmonary system, nitric oxide can combine with oxygen and water to produce nitrogen dioxide and nitrite, respectively, which interact with oxyhemoglobin to produce methemoglobin and nitrate. Thus, the end products of nitric oxide that enter the systemic circulation are predominantly methemoglobin and nitrate.
Metabolism
Methemoglobin disposition has been investigated as a function of time and nitric oxide exposure concentration in neonates with respiratory failure. The methemoglobin (MetHb) concentration-time profiles during the first 12 hours of exposure to 0, 5, 20, and 80 ppm INOmax are shown in Figure 1.
Figure 1: Methemoglobin Concentration-Time Profiles Neonates Inhaling 0, 5, 20 or 80 ppm INOmax
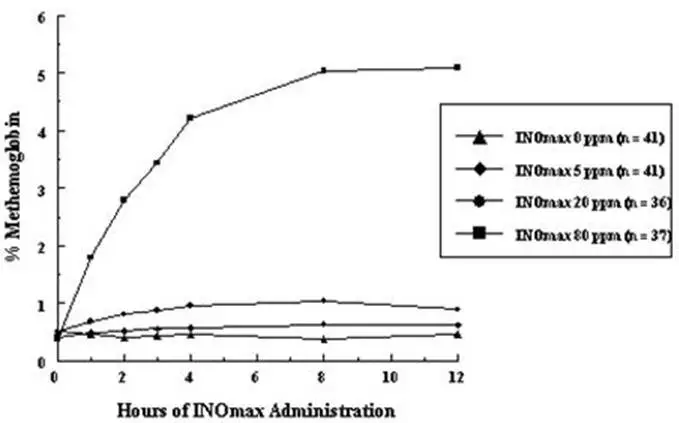
Methemoglobin concentrations increased during the first 8 hours of nitric oxide exposure. The mean methemoglobin level remained below 1% in the placebo group and in the 5 ppm and 20 ppm INOmax groups but reached approximately 5% in the 80 ppm INOmax group. Methemoglobin levels >7% were attained only in patients receiving 80 ppm, where they comprised 35% of the group. The average time to reach peak methemoglobin was 10 ± 9 (SD) hours (median, 8 hours) in these 13 patients, but one patient did not exceed 7% until 40 hours.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of a carcinogenic effect was apparent, at inhalation exposures up to the recommended dose (20 ppm), in rats for 20 hr/day for up to two years. Higher exposures have not been investigated.
Nitric oxide has demonstrated genotoxicity in Salmonella (Ames Test), human lymphocytes, and after in vivo exposure in rats. There are no animal or human studies to evaluate nitric oxide for effects on fertility.
14. Clinical Studies
14.1 Treatment of Hypoxic Respiratory Failure (HRF)
The efficacy of INOmax has been investigated in term and near-term newborns with hypoxic respiratory failure resulting from a variety of etiologies. Inhalation of INOmax reduces the oxygenation index (OI= mean airway pressure in cm H2O × fraction of inspired oxygen concentration [FiO2]× 100 divided by systemic arterial concentration in mm Hg [PaO2]) and increases PaO2 [see Clinical Pharmacology (12.1)].
14.2 Ineffective in Adult Respiratory Distress Syndrome (ARDS)
In a randomized, double-blind, parallel, multicenter study, 385 patients with adult respiratory distress syndrome (ARDS) associated with pneumonia (46%), surgery (33%), multiple trauma (26%), aspiration (23%), pulmonary contusion (18%), and other causes, with PaO2/FiO2 <250 mm Hg despite optimal oxygenation and ventilation, received placebo (n=193) or INOmax (n=192), 5 ppm, for 4 hours to 28 days or until weaned because of improvements in oxygenation. Despite acute improvements in oxygenation, there was no effect of INOmax on the primary endpoint of days alive and off ventilator support. These results were consistent with outcome data from a smaller dose ranging study of nitric oxide (1.25 to 80 ppm). INOmax is not indicated for use in ARDS.
14.3 Ineffective in Prevention of Bronchopulmonary Dysplasia (BPD)
The safety and efficacy of INOmax for the prevention of chronic lung disease [bronchopulmonary dysplasia, (BPD)] in neonates ≤ 34 weeks gestational age requiring respiratory support has been studied in four large, multi-center, double-blind, placebo-controlled clinical trials in a total of 2,600 preterm infants. Of these, 1,290 received placebo, and 1,310 received inhaled nitric oxide at doses ranging from 5-20 ppm, for treatment periods of 7-24 days duration. The primary endpoint for these studies was alive and without BPD at 36 weeks postmenstrual age (PMA). The need for supplemental oxygen at 36 weeks PMA served as a surrogate endpoint for the presence of BPD. Overall, efficacy for the prevention of bronchopulmonary dysplasia in preterm infants was not established. There were no meaningful differences between treatment groups with regard to overall deaths, methemoglobin levels, or adverse events commonly observed in premature infants, including intraventricular hemorrhage, patent ductus arteriosus, pulmonary hemorrhage, and retinopathy of prematurity.
The use of INOmax for prevention of BPD in preterm neonates ≤ 34 weeks gestational age is not recommended.
16. How is Inomax supplied
INOmax (nitric oxide) is available in the following sizes:
| Size D | Portable aluminum cylinders containing 353 liters at STP of nitric oxide gas in 800 ppm concentration in nitrogen (delivered volume 344 liters) (NDC 64693-002-01) |
| Size 88 | Aluminum cylinders containing 1963 liters at STP of nitric oxide gas in 800 ppm concentration in nitrogen (delivered volume 1918 liters) (NDC 64693-002-02) |
| 0.4 liter | Portable aluminum cylinders containing 78 liters at STP of nitric oxide gas in 4,880 ppm concentration in nitrogen (delivered volume 70 liters) (NDC 64693-003-01) |
| INOMAX
nitric oxide gas |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| INOMAX
nitric oxide gas |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - INO THERAPEUTICS LLC (090546628) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Mallinckrodt Manufacturing LLC | 015322416 | MANUFACTURE(64693-002, 64693-003) | |






