Drug Detail:Kalydeco (Ivacaftor [ eye-va-kaf-tor ])
Drug Class: CFTR potentiators
Highlights of Prescribing Information
KALYDECO® (ivacaftor) tablets, for oral use
KALYDECO® (ivacaftor) oral granules
Initial U.S. Approval: 2012
Recent Major Changes
| Indications and Usage (1) | 05/2023 |
| Dosage and Administration (2) | 05/2023 |
| Warnings and Precautions, Hypersensitivity Reactions, Including Anaphylaxis (5.2) | 08/2023 |
Indications and Usage for Kalydeco
KALYDECO is a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator indicated for the treatment of cystic fibrosis (CF) in patients age 1 month and older who have at least one mutation in the CFTR gene that is responsive to ivacaftor based on clinical and/or in vitro assay data. (12.1, 14)
If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use. (1)
Kalydeco Dosage and Administration
| Age | Weight | Dosage | Administration |
|---|---|---|---|
| 1 month to less than 2 months | 3 kg or greater | One 5.8 mg packet every 12 hours | Mixed with one teaspoon (5 ml) of soft food or liquid and administered orally with fat-containing food |
| 2 months to less than 4 months | 3 kg or greater | One 13.4 mg packet every 12 hours | |
| 4 months to less than 6 months | 5 kg or greater | One 25 mg packet every 12 hours | |
| 6 months to less than 6 years | 5 kg to less than 7 kg | One 25 mg packet every 12 hours | |
| 7 kg to less than 14 kg | One 50 mg packet every 12 hours | ||
| 14 kg or greater | One 75 mg packet every 12 hours | ||
| 6 years and older | - | One 150 mg tablet every 12 hours | Taken orally with fat-containing food |
- See full prescribing information for the recommended dosage in patients 6 months and older with moderate or severe hepatic impairment. (2.3, 8.6)
- See full prescribing information for dosage modifications due to drug interactions with KALYDECO. (2.4, 7.1)
- Not recommended in pediatric patients less than 1 month of age. (2.2, 8.4)
- Not recommended in patients 1 month to less than 6 months of age with any level of hepatic impairment and/or taking concomitant moderate or strong CYP3A inhibitors. (2.3, 2.4, 8.6)
Dosage Forms and Strengths
- Tablets: 150 mg (3)
- Oral granules: Unit-dose packets of 5.8 mg, 13.4 mg, 25 mg, 50 mg, and 75 mg (3)
Contraindications
- None (4)
Warnings and Precautions
- Elevated transaminases (ALT or AST): Transaminases (ALT and AST) should be assessed prior to initiating KALYDECO, every 3 months during the first year of treatment, and annually thereafter. In patients with a history of transaminase elevations, more frequent monitoring of liver function tests should be considered. Patients who develop increased transaminase levels should be closely monitored until the abnormalities resolve. Interrupt dosing in patients with ALT or AST of greater than 5 times the upper limit of normal (ULN). Following resolution of transaminase elevations, consider the benefits and risks of resuming KALYDECO dosing. (5.1, 6)
- Hypersensitivity reactions: Anaphylaxis has been reported with KALYDECO in the postmarketing setting. Initiate appropriate therapy in the event of a hypersensitivity reaction. (5.2)
- Use with CYP3A inducers: Concomitant use with strong CYP3A inducers (e.g., rifampin, St. John's wort) substantially decreases exposure of ivacaftor, which may diminish effectiveness. Therefore, co-administration is not recommended. (5.3, 7.2, 12.3)
- Cataracts: Non-congenital lens opacities/cataracts have been reported in pediatric patients treated with KALYDECO. Baseline and follow-up examinations are recommended in pediatric patients initiating KALYDECO treatment. (5.4)
Adverse Reactions/Side Effects
The most common adverse drug reactions to KALYDECO (≥8% of patients with CF who have a G551D mutation in the CFTR gene) were headache, oropharyngeal pain, upper respiratory tract infection, nasal congestion, abdominal pain, nasopharyngitis, diarrhea, rash, nausea, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Vertex Pharmaceuticals Incorporated at 1-877-634-8789 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
CYP3A inhibitors: Reduce KALYDECO dose in patients aged 6 months and older when co-administered with strong CYP3A inhibitors (e.g., ketoconazole) or moderate CYP3A inhibitors (e.g., fluconazole). KALYDECO is not recommended in patients aged 1 month to less than 6 months when co-administered with strong or moderate CYP3A inhibitors. Avoid food or drink containing grapefruit. (2.4, 7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2023
Related/similar drugs
azithromycin, Zithromax, gentamicin, Creon, tobramycinFull Prescribing Information
1. Indications and Usage for Kalydeco
KALYDECO is indicated for the treatment of cystic fibrosis (CF) in patients age 1 month and older who have at least one mutation in the CFTR gene that is responsive to ivacaftor potentiation based on clinical and/or in vitro assay data [see Clinical Pharmacology (12.1) and Clinical Studies (14)].
If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use.
2. Kalydeco Dosage and Administration
2.1 Recommended Dosage in Adults and Pediatric Patients Aged 6 Years and Older
The recommended dosage of KALYDECO for adults and pediatric patients aged 6 years and older is 150 mg orally every 12 hours (300 mg total daily dose) with fat-containing food [see Dosage and Administration (2.5)].
2.2 Recommended Dosage in Pediatric Patients Aged 1 Month to Less than 6 Years
The recommended dosage of KALYDECO (oral granules) for pediatric patients ages 1 month to less than 6 years is weight-based provided in Table 1. Take KALYDECO orally with fat-containing food [see Dosage and Administration (2.5)].
| Age | Body Weight (kg) | KALYDECO Dosage |
|---|---|---|
|
||
| 1 month to less than 2 months*† | 3 kg or greater | One packet (containing 5.8 mg ivacaftor) every 12 hours |
| 2 months to less than 4 months*† | 3 kg or greater | One packet (containing 13.4 mg ivacaftor) every 12 hours |
| 4 months to less than 6 months† | 5 kg or greater | One packet (containing 25 mg ivacaftor) every 12 hours |
| 6 months to less than 6 years of age | 5 kg to less than 7 kg | One packet (containing 25 mg ivacaftor) every 12 hours |
| 7 kg to less than 14 kg | One packet (containing 50 mg ivacaftor) every 12 hours | |
| 14 kg or greater | One packet (containing 75 mg ivacaftor) every 12 hours | |
2.3 Recommended Dosage for Patients with Hepatic Impairment
KALYDECO is not recommended in patients less than 6 months of age with any level of hepatic impairment. The following is the recommended dosage of KALYDECO taken with fat-containing food [see Dosage and Administration (2.5)] for patients aged 6 months and older with hepatic impairment:
-
Mild Hepatic Impairment (Child-Pugh Class A):
- Less than 6 months of age: KALYDECO is not recommended.
- No dose adjustment is necessary for patients aged 6 months or older [see Clinical Pharmacology (12.3)].
-
Moderate Hepatic Impairment (Child-Pugh Class B):
- Less than 6 months of age: KALYDECO is not recommended.
- 6 months to less than 6 years of age: one packet (containing 25 mg, 50 mg, or 75 mg ivacaftor) of oral granules once daily based on dosing recommended for age and weight in Table 1 [see Dosage and Administration (2.2)].
- 6 years of age and older: 150 mg orally once daily.
-
Severe Hepatic Impairment (Child-Pugh Class C): Should not be used in patients less than 6 months of age. In patients 6 months and older should be used with caution. KALYDECO has not been studied in patients with severe hepatic impairment (Child-Pugh Class C), but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a reduced dose, in patients aged 6 months or older with severe hepatic impairment after weighing the risks and benefits of treatment [see Dosage and Administration (2.1, 2.2) and Clinical Pharmacology (12.3)].
- Less than 6 months of age: KALYDECO is not recommended.
- 6 months to less than 6 years of age: one packet (containing 25 mg, 50 mg, or 75 mg ivacaftor) of oral granules once daily or less frequently based on dosing recommended for age and weight in Table 1 [see Dosage and Administration (2.2)].
- 6 years of age and older: 150 mg orally once daily or less frequently.
2.4 Dosage Modification for Patients Taking Drugs that are CYP3A Inhibitors
Concomitant use of moderate or strong CYP3A inhibitors is not recommended in patients below 6 months of age. Food or drink containing grapefruit should be avoided [see Drug Interactions (7.1), Clinical Pharmacology (12.3)]. Take KALYDECO with fat-containing food [see Dosage and Administration (2.5)].
3. Dosage Forms and Strengths
Tablets: 150 mg, light blue, film-coated, capsule shaped tablets, with the characters "V 150" on one side and plain on the other.
Oral granules: 5.8 mg, 13.4 mg, 25 mg, 50 mg, or 75 mg, white to off-white granules, in unit-dose packets.
5. Warnings and Precautions
5.1 Transaminase (ALT or AST) Elevations
Elevated transaminases have been reported in patients with CF receiving KALYDECO. ALT and AST should be assessed prior to initiating KALYDECO, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of transaminase elevations, consider more frequent monitoring of liver function tests. Patients who develop increased transaminase levels should be closely monitored until the abnormalities resolve. Dosing should be interrupted in patients with ALT or AST of greater than 5 times the upper limit of normal (ULN). Following resolution of transaminase elevations, consider the benefits and risks of resuming KALYDECO [see Adverse Reactions (6) and Use in Specific Populations (8.6)].
5.2 Hypersensitivity Reactions, Including Anaphylaxis
Hypersensitivity reactions, including cases of anaphylaxis, have been reported in the postmarketing setting [see Adverse Reactions (6.2)]. If signs or symptoms of serious hypersensitivity reactions develop during treatment, discontinue KALYDECO and institute appropriate therapy. Consider the benefits and risks for the individual patient to determine whether to resume treatment with KALYDECO.
5.3 Concomitant Use with CYP3A Inducers
Use of KALYDECO with strong CYP3A inducers, such as rifampin, substantially decreases the exposure of ivacaftor, which may reduce the therapeutic effectiveness of KALYDECO. Therefore, co-administration of KALYDECO with strong CYP3A inducers (e.g., rifampin, St. John's wort) is not recommended [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)].
5.4 Cataracts
Cases of non-congenital lens opacities/cataracts have been reported in pediatric patients treated with KALYDECO. Although other risk factors were present in some cases (such as corticosteroid use and/or exposure to radiation), a possible risk attributable to KALYDECO cannot be excluded. Baseline and follow-up ophthalmological examinations are recommended in pediatric patients initiating KALYDECO treatment.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Transaminase Elevations [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions, Including Anaphylaxis [see Warnings and Precautions (5.2)]
- Cataracts [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The overall safety profile of KALYDECO is based on pooled data from three placebo-controlled clinical trials conducted in 353 patients 6 years of age and older with CF who had a G551D mutation in the CFTR gene (Trials 1 and 2) or were homozygous for the F508del mutation (Trial 3). In addition, the following clinical trials have also been conducted [see Clinical Pharmacology (12) and Clinical Studies (14)]:
- An 8-week, crossover design trial (Trial 4) involving 39 patients between the ages of 6 and 57 years with a G1244E, G1349D, G178R, G551S, G970R, S1251N, S1255P, S549N, or S549R mutation in the CFTR gene.
- A 24-week, placebo-controlled trial (Trial 5) involving 69 patients between the ages of 6 and 68 years with an R117H mutation in the CFTR gene.
- A 24-week, open-label trial (Trial 6) in 34 patients 2 to less than 6 years of age. Patients eligible for Trial 6 were those with the G551D, G1244E, G1349D, G178R, G551S, G970R, S1251N, S1255P, S549N, or S549R mutation in the CFTR gene. Of 34 patients enrolled, 32 had the G551D mutation and 2 had the S549N mutation.
- An 8-week, crossover design trial (Trial 7) involving patients between the ages of 12 and 72 years who were heterozygous for the F508del mutation and a second CFTR mutation predicted to be responsive to ivacaftor. A total of 156 patients were randomized to and received KALYDECO.
- A 24-week open-label clinical trial in patients with CF aged less than 24 months (Trial 8) including a cohort of 19 patients aged 12 months to less than 24 months, a cohort of 11 patients aged 6 months to less than 12 months, a cohort of 6 patients aged 4 months to less than 6 months, and a cohort of 7 patients aged 1 month to less than 4 months. Patients with a gating mutation or R117H mutation were eligible for the first three cohorts of this study. Patients with any ivacaftor-responsive mutation were eligible for the cohort aged 1 to less than 4 months.
Of the 353 patients included in the pooled analyses of patients with CF who had either a G551D mutation or were homozygous for the F508del mutation in the CFTR gene, 50% of patients were female and 97% were Caucasian; 221 received KALYDECO, and 132 received placebo for 16 to 48 weeks.
The proportion of patients who prematurely discontinued study drug due to adverse reactions was 2% for KALYDECO-treated patients and 5% for placebo-treated patients. Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in KALYDECO-treated patients, included abdominal pain, increased hepatic enzymes, and hypoglycemia.
The most common adverse reactions in the 221 patients treated with KALYDECO were headache (17%), upper respiratory tract infection (16%), nasal congestion (16%), nausea (10%), rash (10%), rhinitis (6%), dizziness (5%), arthralgia (5%), and bacteria in sputum (5%).
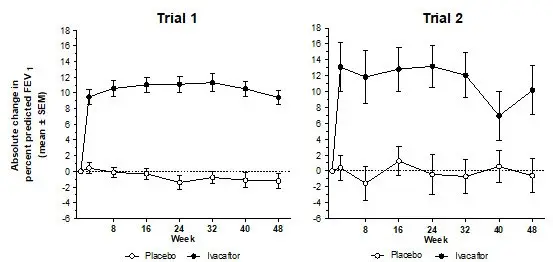
The incidence of adverse reactions below is based upon two double-blind, placebo-controlled, 48-week clinical trials (Trials 1 and 2) in a total of 213 patients with CF ages 6 to 53 who have a G551D mutation in the CFTR gene and who were treated with KALYDECO 150 mg orally or placebo twice daily. Table 2 shows adverse reactions occurring in ≥8% of KALYDECO-treated patients with CF who have a G551D mutation in the CFTR gene that also occurred at a higher rate than in the placebo-treated patients in the two double-blind, placebo-controlled trials.
| Adverse Reaction (Preferred Term) | Incidence: Pooled 48-Week Trials | |
|---|---|---|
| KALYDECO N=109 n (%) | Placebo N=104 n (%) |
|
| Headache | 26 (24) | 17 (16) |
| Oropharyngeal pain | 24 (22) | 19 (18) |
| Upper respiratory tract infection | 24 (22) | 14 (14) |
| Nasal congestion | 22 (20) | 16 (15) |
| Abdominal pain | 17 (16) | 13 (13) |
| Nasopharyngitis | 16 (15) | 12 (12) |
| Diarrhea | 14 (13) | 10 (10) |
| Rash | 14 (13) | 7 (7) |
| Nausea | 13 (12) | 11 (11) |
| Dizziness | 10 (9) | 1 (1) |
Adverse reactions in the 48-week clinical trials that occurred in the KALYDECO group at a frequency of 4 to 7% where rates exceeded that in the placebo group include:
- Infections and infestations: rhinitis
- Investigations: aspartate aminotransferase increased, bacteria in sputum, blood glucose increased, hepatic enzyme increased
- Musculoskeletal and connective tissue disorders: arthralgia, musculoskeletal chest pain, myalgia
- Nervous system disorders: sinus headache
- Respiratory, thoracic and mediastinal disorders: pharyngeal erythema, pleuritic pain, sinus congestion, wheezing
- Skin and subcutaneous tissue disorders: acne
The safety profile for the CF patients enrolled in the other clinical trials (Trials 3-8) was similar to that observed in the 48-week, placebo-controlled trials (Trials 1 and 2).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of KALYDECO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: anaphylaxis
7. Drug Interactions
Potential for other drugs to affect ivacaftor
7.1 Inhibitors of CYP3A
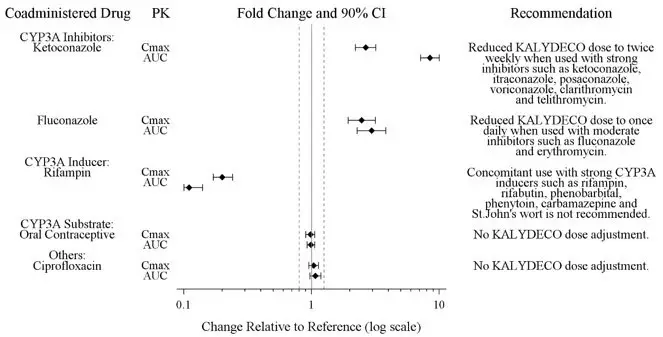
Ivacaftor is a sensitive CYP3A substrate. Co-administration with ketoconazole, a strong CYP3A inhibitor, significantly increased ivacaftor exposure [measured as area under the curve (AUC)] by 8.5-fold. Based on simulations of these results, a reduction of the KALYDECO dose is recommended for patients 6 months and older taking concomitant strong CYP3A inhibitors, such as ketoconazole, itraconazole, posaconazole, voriconazole, telithromycin, and clarithromycin. KALYDECO is not recommended for patients less than 6 months of age taking strong CYP3A inhibitors [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
Co-administration with fluconazole, a moderate inhibitor of CYP3A, increased ivacaftor exposure by 3-fold. Therefore, a reduction of the KALYDECO dose is recommended for patients 6 months and older taking concomitant moderate CYP3A inhibitors, such as fluconazole and erythromycin. KALYDECO is not recommended for patients less than 6 months of age taking moderate CYP3A inhibitors [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
Co-administration of KALYDECO with grapefruit juice, which contains one or more components that moderately inhibit CYP3A, may increase exposure of ivacaftor. Therefore, avoid food or drink containing grapefruit during treatment with KALYDECO [see Clinical Pharmacology (12.3)].
7.2 Inducers of CYP3A
Co-administration with rifampin, a strong CYP3A inducer, significantly decreased ivacaftor exposure (AUC) by approximately 9-fold. Therefore, co-administration with strong CYP3A inducers, such as rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John's wort is not recommended [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
7.3 Ciprofloxacin
Co-administration of KALYDECO with ciprofloxacin had no effect on the exposure of ivacaftor. Therefore, no dose adjustment is necessary during concomitant administration of KALYDECO with ciprofloxacin [see Clinical Pharmacology (12.3)].
Potential for ivacaftor to affect other drugs
7.4 CYP2C9 Substrates
Ivacaftor may inhibit CYP2C9; therefore, monitoring of the international normalized ratio (INR) during co-administration of KALYDECO with warfarin is recommended. Other therapeutic products for which exposure may be increased by KALYDECO include glimepiride and glipizide; these therapeutic products should be used with caution [see Clinical Pharmacology (12.3)].
7.5 CYP3A and/or P-gp Substrates
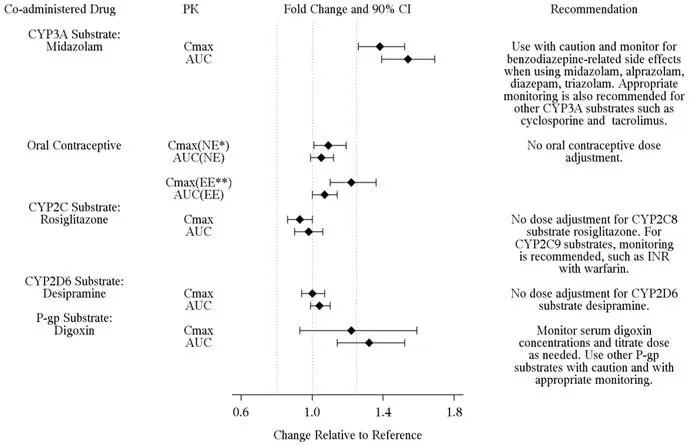
Ivacaftor and its M1 metabolite have the potential to inhibit CYP3A and P-gp. Co-administration with oral midazolam, a sensitive CYP3A substrate, increased midazolam exposure 1.5-fold, consistent with weak inhibition of CYP3A by ivacaftor. Co-administration with digoxin, a sensitive P-gp substrate, increased digoxin exposure by 1.3-fold, consistent with weak inhibition of P-gp by ivacaftor. Administration of KALYDECO may increase systemic exposure of drugs that are substrates of CYP3A and/or P-gp, which may increase or prolong their therapeutic effect and adverse events. Therefore, caution and appropriate monitoring are recommended when co-administering KALYDECO with sensitive CYP3A and/or P-gp substrates, such as digoxin, cyclosporine, and tacrolimus [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of KALYDECO for the treatment of CF have been established in pediatric patients 1 month to 17 years of age who have at least one mutation in the CFTR gene that is responsive to ivacaftor potentiation based on clinical and/or in vitro assay data [see Clinical Pharmacology (12.1) and Clinical Studies (14)].
The use of KALYDECO for this indication is supported by evidence from placebo-controlled clinical trials in the following pediatric patients with CF:
- 6 to 17 years of age with a G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, S549R, or R117H mutation in the CFTR gene [see Adverse Reactions (6) and Clinical Studies (14)].
- 12 to 17 years of age who are heterozygous for the F508del mutation and a second mutation predicted to be responsive to ivacaftor [see Adverse Reactions (6) and Clinical Studies (14)].
The effectiveness of KALYDECO in patients aged 2 to less than 6 years was extrapolated from patients 6 years of age and older with support from population pharmacokinetic analyses showing similar drug exposure levels in adults and pediatric patients 2 to less than 6 years of age [see Clinical Pharmacology (12.3)]. Safety of KALYDECO in this population was derived from a 24-week, open-label clinical trial in 34 patients ages 2 to less than 6 years (mean age 3 years) administered either 50 mg or 75 mg of ivacaftor granules twice daily (Trial 6). The type and frequency of adverse reactions in this trial were similar to those in patients 6 years and older. Transaminase elevations were more common in patients who had abnormal transaminases at baseline [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
The effectiveness of KALYDECO in patients aged 1 month to less than 24 months was extrapolated from patients 6 years of age and older with support from population pharmacokinetic analyses showing that the exposure of ivacaftor in pediatric patients 1 month to less than 24 months of age is within the range of exposure in adults and pediatric patients 6 years of age and older [see Clinical Pharmacology (12.3)]. Safety of KALYDECO in this population was derived from a cohort of 7 patients aged 1 month to less than 4 months (mean age 1.9 months at baseline), a cohort of 6 patients aged 4 months to less than 6 months (mean age 4.5 months at baseline), a cohort of 11 patients aged 6 months to less than 12 months (mean age 9.0 months at baseline), and a cohort of 19 patients aged 12 months to less than 24 months (mean age 15.2 months at baseline) in a 24-week, open-label clinical trial, administered 5.8 mg, 11.4 mg, 17.1 mg, 22.8 mg, 25 mg, 50 mg, or 75 mg (11.4 mg, 17.1 mg, and 22.8 mg are not recommended dosages) of ivacaftor granules twice daily (Trial 8). The safety profile of patients in this trial was similar to that observed in patients 2 years and older.
The safety and effectiveness of KALYDECO in pediatric patients with CF younger than 1 month of age have not been established.
8.5 Geriatric Use
CF is largely a disease of children and young adults. Clinical trials of KALYDECO did not include sufficient numbers of patients 65 years of age and over to determine whether they respond differently from younger patients.
8.6 Hepatic Impairment
- Mild Hepatic Impairment (Child-Pugh Class A): No dose adjustment is necessary for patients aged 6 months or older [see Clinical Pharmacology (12.3)].
- Moderate Hepatic Impairment (Child-Pugh Class B): A reduced dose is recommended in patients aged 6 months or older [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
- Severe Hepatic Impairment (Child-Pugh Class C): Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C), but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a reduced dose, in patients aged 6 months or older with severe hepatic impairment after weighing the risks and benefits of treatment [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
Due to variability in maturation of cytochrome (CYP) enzymes involved in ivacaftor metabolism, treatment with KALYDECO is not recommended in patients aged 1 month to less than 6 months with any level of hepatic impairment [see Dosage and Administration (2.3)].
8.7 Renal Impairment
KALYDECO has not been studied in patients with mild, moderate, or severe renal impairment or in patients with end-stage renal disease. No dose adjustment is necessary for patients with mild to moderate renal impairment; however, caution is recommended while using KALYDECO in patients with severe renal impairment (creatinine clearance less than or equal to 30 mL/min) or end-stage renal disease.
10. Overdosage
There have been no reports of overdose with KALYDECO.
No specific antidote is available for overdose with KALYDECO. Treatment of overdose with KALYDECO consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
11. Kalydeco Description
The active ingredient in KALYDECO tablets and oral granules is ivacaftor, a cystic fibrosis transmembrane conductance regulator potentiator, which has the following chemical name: N-(2,4-di-tert-butyl-5-hydroxyphenyl)-1,4-dihydro-4-oxoquinoline-3-carboxamide. Its molecular formula is C24H28N2O3 and its molecular weight is 392.49. Ivacaftor has the following structural formula:
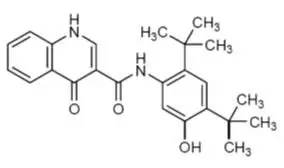
Ivacaftor is a white to off-white powder that is practically insoluble in water (<0.05 microgram/mL).
KALYDECO is available as a light blue, capsule shaped, film-coated tablet for oral administration containing 150 mg of ivacaftor. Each KALYDECO tablet contains 150 mg of ivacaftor and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The tablet film coat contains carnauba wax, FD&C Blue #2, PEG 3350, polyvinyl alcohol, talc, and titanium dioxide. The printing ink contains ammonium hydroxide, iron oxide black, propylene glycol, and shellac.
KALYDECO is also available as white to off-white granules for oral administration (sweetened but unflavored) and enclosed in a unit-dose packet containing 5.8 mg of ivacaftor, 13.4 mg of ivacaftor, 25 mg of ivacaftor, 50 mg of ivacaftor, or 75 mg of ivacaftor. Each unit-dose packet of KALYDECO oral granules contains 5.8 mg of ivacaftor, 13.4 mg of ivacaftor, 25 mg of ivacaftor, 50 mg of ivacaftor, or 75 mg of ivacaftor and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, mannitol, sucralose, and sodium lauryl sulfate.
12. Kalydeco - Clinical Pharmacology
12.1 Mechanism of Action
Ivacaftor is a potentiator of the CFTR protein. The CFTR protein is a chloride channel present at the surface of epithelial cells in multiple organs. Ivacaftor facilitates increased chloride transport by potentiating the channel open probability (or gating) of CFTR protein located at the cell surface. The overall level of ivacaftor-mediated CFTR chloride transport is dependent on the amount of CFTR protein at the cell surface and how responsive a particular mutant CFTR protein is to ivacaftor potentiation.
12.3 Pharmacokinetics
The pharmacokinetics of ivacaftor is similar between healthy adult volunteers and patients with CF.
After oral administration of a single 150 mg dose to healthy volunteers in a fed state, peak plasma concentrations (Tmax) occurred at approximately 4 hours, and the mean (±SD) for AUC and Cmax were 10600 (5260) ng*hr/mL and 768 (233) ng/mL, respectively.
After every 12-hour dosing, steady-state plasma concentrations of ivacaftor were reached by days 3 to 5, with an accumulation ratio ranging from 2.2 to 2.9.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year studies were conducted in CD-1 mice and Sprague-Dawley rats to assess carcinogenic potential of KALYDECO. No evidence of tumorigenicity was observed in mice or rats at ivacaftor oral doses up to 200 mg/kg/day and 50 mg/kg/day, respectively (approximately equal to 1 and 4 times the MRHD based on summed AUCs of ivacaftor and its metabolites).
Ivacaftor was negative for genotoxicity in the following assays: Ames test for bacterial gene mutation, in vitro chromosomal aberration assay in Chinese hamster ovary cells, and in vivo mouse micronucleus test.
Ivacaftor impaired fertility and reproductive performance indices in male and female rats at 200 mg/kg/day (yielding exposures approximately 8 and 5 times, respectively, the MRHD based on summed AUCs of ivacaftor and its major metabolites). Increases in prolonged diestrus were observed in females at 200 mg/kg/day. Ivacaftor also increased the number of females with all nonviable embryos and decreased corpora lutea, implantations, and viable embryos in rats at 200 mg/kg/day (approximately 5 times the MRHD based on summed AUCs of ivacaftor and its major metabolites) when dams were dosed prior to and during early pregnancy. These impairments of fertility and reproductive performance in male and female rats at 200 mg/kg/day were attributed to severe toxicity. No effects on male or female fertility and reproductive performance indices were observed at ≤100 mg/kg/day (yielding exposures approximately 6 and 3 times, respectively, the MRHD based on summed AUCs of ivacaftor and its major metabolites).
14. Clinical Studies
14.2 Trial in Patients with a G1244E, G1349D, G178R, G551S, G970R, S1251N, S1255P, S549N, or S549R Mutation in the CFTR Gene
The efficacy and safety of KALYDECO in patients with CF who have a G1244E, G1349D, G178R, G551S, G970R, S1251N, S1255P, S549N, or S549R mutation in the CFTR gene were evaluated in a two-part, randomized, double-blind, placebo-controlled, crossover design clinical trial in 39 patients with CF (Trial 4). Patients who completed Part 1 of this trial continued into the 16-week open-label Part 2 of the study. The mutations studied were G178R, S549N, S549R, G551S, G970R, G1244E, S1251N, S1255P, and G1349D. See Clinical Studies (14.1) for efficacy in patients with a G551D mutation.
Patients were 6 years of age or older (mean age 23 years) with FEV1 ≥40% at screening [mean FEV1 at baseline 78% predicted (range: 43% to 119%)]. Patients with evidence of colonization with Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus and those with abnormal liver function defined as 3 or more liver function tests (ALT, AST, AP, GGT, total bilirubin) ≥3 times the ULN at screening were excluded.
Patients were randomized 1:1 to receive either 150 mg of KALYDECO or placebo every 12 hours with fat-containing food for 8 weeks in addition to their prescribed CF therapies during the first treatment period and crossed over to the other treatment for the second 8 weeks. The two 8-week treatment periods were separated by a 4- to 8-week washout period. The use of inhaled hypertonic saline was not permitted.
The primary efficacy endpoint was improvement in lung function as determined by the mean absolute change from baseline in percent predicted FEV1 through 8 weeks of treatment. Other efficacy variables included absolute change from baseline in sweat chloride through 8 weeks of treatment [see Clinical Pharmacology (12.2)], absolute change from baseline in body mass index (BMI) at 8 weeks of treatment (including body weight at 8 weeks), and improvement in CFQ-R respiratory domain score through 8 weeks of treatment. For the overall population of the 9 mutations studied, treatment with KALYDECO compared to placebo resulted in significant improvement in percent predicted FEV1 [10.7 through Week 8 (P<0.0001)], BMI [0.66 kg/m2 at Week 8 (P<0.0001)], and CFQ-R respiratory domain score [9.6 through Week 8 (P=0.0004)]; however, there was a high degree of variability of efficacy responses among the 9 mutations (Table 6).
| Mutation (n) | Absolute change in percent predicted FEV1 | BMI (kg/m2) | CFQ-R Respiratory Domain Score (Points) | Absolute Change in Sweat Chloride (mmol/L) |
||
|---|---|---|---|---|---|---|
| At Week 2 | At Week 4 | At Week 8 | At Week 8 | At Week 8 | At Week 8 | |
|
||||||
| All patients (n=39) Results shown as mean (95% CI) change from baseline KALYDECO vs. placebo-treated patients: |
||||||
| 8.3 (4.5, 12.1) | 10.0 (6.2, 13.8) | 13.8 (9.9, 17.6) | 0.66 * (0.34, 0.99) | 12.8 (6.7, 18.9) | -50 (-58, -41) † | |
| Patients grouped under mutation types (n)
Results shown as mean (minimum, maximum) for change from baseline for KALYDECO-treated patients ‡: |
||||||
| G1244E (5) | 11 (-5, 25) | 6 (-5, 13) | 8 (-1, 18) | 0.63 (0.34, 1.32) | 3.3 (-27.8, 22.2) | -55 (-75, -34) |
| G1349D (2) | 19 (5, 33) | 18 (2, 35) | 20 (3, 36) | 1.15 (1.07, 1.22) | 16.7 (-11.1, 44.4) | -80 (-82, -79) |
| G178R (5) | 7 (1, 17) | 10 (-2, 21) | 8 (-1, 18) | 0.85 (0.33, 1.46) | 20.0 (5.6, 50.0) | -53 (-65, -35) |
| G551S (2) | 0 (-5, 5) | 0.3 (-5, 6) | 3 § | 0.16 § | 16.7 § | -68 § |
| G970R (4) | 7 (1, 13) | 7 (1, 14) | 3 (-1, 5) | 0.48 (-0.38, 1.75) | 1.4 (-16.7, 16.7) | -6 (-16, -2) |
| S1251N (8) | 2 (-23, 20) | 8 (-13, 26) | 9 (-20, 21) | 0.73 (0.08, 1.83) | 23.3 (5.6, 50.0) | -54 (-84, -7) |
| S1255P (2) | 11 (8, 14) | 9 (5, 13) | 3 (-1, 8) | 1.62 (1.39, 1.84) | 8.3 (5.6, 11.1) | -78 (-82, -74) |
| S549N (6) | 11 (5, 16) | 8 (-9, 19) | 11 (-2, 20) | 0.79 (0.00, 1.91) | 8.8 (-8.3, 27.8) | -74 (-93, -53) |
| S549R (4) | 3 (-4, 8) | 4 (-4, 10) | 5 (-3, 13) | 0.53 (0.33, 0.80) | 6.9 (0.0, 11.1) | -61 ¶ (-71, -54) |
14.3 Trial in Patients with CF who have an R117H Mutation in the CFTR Gene
The efficacy and safety of KALYDECO in patients with CF who have an R117H mutation in the CFTR gene were evaluated in a randomized, double-blind, placebo-controlled, parallel-group clinical trial (Trial 5). Fifty-nine of 69 patients completed 24 weeks of treatment. Two patients discontinued and 8 patients did not complete treatment due to study termination. Trial 5 evaluated 69 clinically stable patients with CF who were 6 years of age or older (mean age 31 years). Patients who were 12 years and older had FEV1 at screening between 40-90% predicted, and patients who were 6-11 years of age had FEV1 at screening between 40-105% predicted. The overall mean FEV1 was 73% predicted at baseline (range: 33% to 106%). The patients had well preserved BMIs (mean overall: 23.76 kg/m2) and a high proportion were pancreatic sufficient as assessed by a low rate of pancreatic enzyme replacement therapy use (pancreatin: 11.6%; pancrelipase: 5.8%). Patients who had persistent Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus isolated from sputum at screening, and those with abnormal liver function defined as 3 or more liver function tests (ALT, AST, AP, GGT, total bilirubin) ≥3 times the ULN, were excluded.
Patients were randomized 1:1 to receive either 150 mg of KALYDECO (n=34) or placebo (n=35) every 12 hours with fat-containing food for 24 weeks in addition to their prescribed CF therapies.
The primary efficacy endpoint was improvement in lung function as determined by the mean absolute change from baseline in percent predicted FEV1 through 24 weeks of treatment. The treatment difference for absolute change in percent predicted FEV1 through Week 24 was 2.1 percentage points (analysis conducted with the full analysis set which included all 69 patients) and did not reach statistical significance (Table 7).
Other efficacy variables that were analyzed included absolute change in sweat chloride from baseline through Week 24, improvement in cystic fibrosis respiratory symptoms through Week 24 as assessed by the CFQ-R respiratory domain score (Table 7), absolute change in body mass index (BMI) at Week 24, and time to first pulmonary exacerbation. The overall treatment difference for the absolute change from baseline in BMI at Week 24 was 0.3 kg/m2 and the calculated hazard ratio for time to first pulmonary exacerbation was 0.93, which were not statistically significant.
Statistically significant improvements in clinical efficacy (FEV1, CFQ-R respiratory domain score) were seen in several subgroup analyses and decreases in sweat chloride were observed in all subgroups. The mean baseline sweat chloride for all patients was 70 mmol/L. Subgroups analyzed included those based on age, lung function, and poly-T status (Table 7).
| Absolute Change through Week 24 *- All Randomized Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Predicted FEV1
(Percentage Points) | CFQ-R Respiratory Domain Score (Points) | Sweat Chloride (mmol/L) |
||||||||
| Subgroup Parameter | Study Drug | n | Mean | Treatment Difference (95% CI) | n | Mean | Treatment Difference (95% CI) | n | Mean | Treatment Difference (95% CI) |
|
||||||||||
| R117H–All Patients | ||||||||||
| Placebo KALYDECO | 35 34 | 0.5 2.6 | 2.1 (-1.1, 5.4) | 34 33 | -0.8 7.6 | 8.4 (2.2, 14.6) | 35 32 | -2.3 -26.3 | -24.0 (-28.0, -19.9) |
|
| Subgroup by Age | ||||||||||
| 6-11 | Placebo KALYDECO | 8 9 | 3.5 -2.8 | -6.3 (-12.0, -0.7) | 7 8 | -1.6 -7.7 | -6.1 (-15.7, 3.4) | 8 8 | 1.0 -26.6 | -27.6 (-37.2, -18.1) |
| 12-17 | Placebo KALYDECO | 1 1 | --- | --- | 1 1 | --- | --- | 1 1 | --- | --- |
| ≥18 | Placebo KALYDECO | 26 24 | -0.5 4.5 | 5.0 (1.1, 8.8) | 26 24 | -0.5 12.2 | 12.6 (5.0, 20.3) | 26 23 | -4.0 -25.9 | -21.9 (-26.5, -17.3) |
| Subgroup by Poly-T Status † | ||||||||||
| 5T | Placebo KALYDECO | 24 14 | 0.7 6.0 | 5.3 (1.3, 9.3) | 24 14 | -0.6 14.7 | 15.3 (7.7, 23.0) | 24 13 | -4.6 -28.7 | -24.2 (-30.2, -18.2) |
| 7T | Placebo KALYDECO | 5 11 | -0.9 -0.7 | 0.2 (-8.1, 8.5) | 5 11 | -6.0 -0.7 | 5.2 (-13.0, 23.4) | 5 10 | 3.9 -20.2 | -24.1 (-33.9, -14.3) |
| Subgroup by Baseline FEV1 % Predicted | ||||||||||
| <70% | Placebo KALYDECO | 15 13 | 0.4 4.5 | 4.0 (-2.1, 10.1) | 15 13 | 3.0 14.4 | 11.4 (1.2, 21.6) | 15 12 | -3.8 -29.3 | -25.5 (-31.8, -19.3) |
| 70-90% | Placebo KALYDECO | 14 14 | 0.2 2.8 | 2.6 (-2.3, 7.5) | 13 14 | -3.6 5.2 | 8.8 (-2.6, 20.2) | 14 14 | -3.1 -23.0 | -20.0 (-26.9, -12.9) |
| >90% | Placebo KALYDECO | 6 7 | 2.2 -2.1 | -4.3 (-9.9, 1.3) | 6 6 | -2.5 -3.2 | -0.7 (-10.4, 9.0) | 6 6 | 1.0 -25.9 | -26.8 (-39.5, -14.1) |
14.4 Trial in Patients with CF Heterozygous for the F508del Mutation and a Second Mutation Predicted to be Responsive to ivacaftor
The efficacy and safety of KALYDECO and an ivacaftor-containing combination product in 246 patients with CF was evaluated in a randomized, double-blind, placebo-controlled, 2-period, 3-treatment, 8-week crossover design clinical trial (Trial 7). Mutations predicted to be responsive to ivacaftor were selected for the study based on the clinical phenotype (pancreatic sufficiency), biomarker data (sweat chloride), and in vitro responsiveness to ivacaftor.
Eligible patients were heterozygous for the F508del mutation with a second mutation predicted to be responsive to ivacaftor. Of the 244 patients included in the efficacy analysis, who were randomized and dosed, 146 patients had a splice mutation and 98 patients had a missense mutation, as the second allele. 156 patients received KALYDECO and 161 patients received placebo. Patients were aged 12 years and older (mean age 35 years [range 12-72]) and had a percent predicted FEV1 at screening between 40-90 [mean ppFEV1 at study baseline 62 (range: 35 to 94)]. Patients with evidence of colonization with organisms associated with a more rapid decline in pulmonary status (e.g., Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus) and those with abnormal liver function at screening were excluded. Abnormal liver function was defined as 2 or more liver function tests (ALT, AST, ALP, GGT) ≥3 times the ULN or total bilirubin ≥2 times the ULN, or a single increase in ALT/AST ≥5 times the ULN.
The primary efficacy endpoint was the mean absolute change from study baseline in percent predicted FEV1 averaged at Weeks 4 and 8 of treatment. The key secondary efficacy endpoint was absolute change in CFQ-R respiratory domain score from study baseline averaged at Weeks 4 and 8 of treatment. For the overall population, treatment with KALYDECO compared to placebo resulted in significant improvement in ppFEV1 [4.7 percent points from study baseline to average of Week 4 and Week 8 (P<0.0001)] and CFQ-R respiratory domain score [9.7 points from study baseline to average of Week 4 and Week 8 (P<0.0001)]. Statistically significant improvements compared to placebo were also observed in the subgroup of patients with splice mutations and missense mutations (Table 8).
| Mutation (n) | Absolute Change in percent predicted FEV1 *† | Absolute Change in CFQ-R Respiratory Domain Score (Points) *‡ | Absolute Change in Sweat Chloride (mmol/L) *‡ |
|---|---|---|---|
|
|||
| Splice mutations (n=94 for IVA and n=97 for PBO) Results shown as difference in mean (95% CI) change from study baseline for KALYDECO vs. placebo-treated patients: |
|||
| 5.4 (4.1, 6.8) | 8.5 (5.3, 11.7) | -2.4 (-5.0, 0.3) |
|
| By individual splice mutation (n). Results shown as mean (minimum, maximum) for change from study baseline for KALYDECO-treated patients | |||
| 2789+5G→A (28) | 5.1 (-7.1, 17.0) | 8.6 (-5.6, 27.8) | 0.4 (-7.5, 8.8) |
| 3272-26A→G (23) | 3.5 (-9.1, 16.0) | 8.0 (-11.1, 27.8) | -2.3 (-25.0, 11.8) |
| 3849+10kbC→T (40) | 5.1 (-6.8, 16.2) | 7.5 (-30.6, 55.6) | -4.6 (-80.5, 23.0) |
| 711+3A→G (2) | 9.2 (8.9, 9.6) | -8.3 (-13.9, -2.8) | -9.9 (-13.5, -6.3) |
| E831X (1) | 7.1 (7.1, 7.1) | 0.0 (0.0, 0.0) | -7.8 (-7.8, -7.8) |
| Missense mutations (n=62 for IVA and n=63 for PBO) Results shown as difference in mean (95% CI) change from study baseline for KALYDECO vs. placebo-treated patients: |
|||
| 3.6 (1.9, 5.2) | 11.5 (7.5, 15.4) | -7.8 (-11.2, -4.5) |
|
| By individual missense mutation (n). Results shown as mean (minimum, maximum) for change from study baseline for KALYDECO-treated patients | |||
| D579G (2) | 13.3 (12.4, 14.1) | 15.3 (-2.8, 33.3) | -30.8 (-36.0, -25.5) |
| D1152H (15) | 2.4 (-5.0, 10.2) | 13.7 (-16.7, 50.0) | -4.8 (-22.0, 3.0) |
| A455E (14) | 3.7 (-6.6, 19.7) | 6.8 (-13.9, 33.3) | 7.5 (-16.8, 16.0) |
| L206W (2) | 4.2 (2.5, 5.9) | 12.5 (-5.6, 30.6) | 3.9 (-8.3, 16.0) |
| P67L (12) | 4.3 (-2.5, 25.7) | 10.8 (-12.5, 36.1) | -10.5 (-34.8, 9.8) |
| R1070W (1) | 2.9 (2.9, 2.9) | 44.4 (44.4, 44.4) | 0.3 (0.3, 0.3) |
| R117C (1) | 3.5 (3.5, 3.5) | 22.2 (22.2, 22.2) | -36.0 (-36.0, -36.0) |
| R347H (3) | 2.5 (-0.6, 6.9) | 6.5 (5.6, 8.3) | -19.2 (-25.8, -7.0) |
| R352Q (2) | 4.4 (3.5, 5.3) | 9.7 (8.3, 11.1) | -21.9 (-45.5, 1.8) |
| S945L (9) | 8.8 (-0.2, 20.5) | 10.6 (-25.0, 27.8) | -30.8 (-50.8, -17.3) |
| S977F (1) | 4.3 (4.3, 4.3) | -2.8 (-2.8, -2.8) | -19.5 (-19.5, -19.5) |
In an analysis of BMI at Week 8, an exploratory endpoint, patients treated with KALYDECO had a mean improvement of 0.28 kg/m2 [95% CI (0.14, 0.43)], 0.24 kg/m2 [95% CI (0.06, 0.43)], and 0.35 kg/m2 [95% CI (0.12, 0.58)] versus placebo for the overall, splice, and missense mutation populations of patients, respectively.
14.5 Trial in Patients Homozygous for the F508del Mutation in the CFTR Gene
Trial 3 was a 16-week, randomized, double-blind, placebo-controlled, parallel-group trial in 140 patients with CF age 12 years and older who were homozygous for the F508del mutation in the CFTR gene and who had FEV1 ≥40% predicted. Patients were randomized 4:1 to receive KALYDECO 150 mg (n=112) every 12 hours or placebo (n=28) in addition to their prescribed CF therapies. The mean age of patients enrolled was 23 years and the mean baseline FEV1 was 79% predicted (range 40% to 129%). As in Trials 1 and 2, patients who had persistent Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus isolated from sputum at screening and those with abnormal liver function defined as 3 or more liver function tests (ALT, AST, AP, GGT, total bilirubin) ≥3 times the ULN were excluded. The use of inhaled hypertonic saline was not permitted.
The primary endpoint was improvement in lung function as determined by the mean absolute change from baseline through Week 16 in percent predicted FEV1. The treatment difference from placebo for the mean absolute change in percent predicted FEV1 through Week 16 in patients with CF homozygous for the F508del mutation in the CFTR gene was 1.72 percentage points (1.5% and -0.2% for patients in the KALYDECO and placebo-treated groups, respectively) and did not reach statistical significance (Table 9).
Other efficacy variables that were analyzed included absolute change in sweat chloride from baseline through Week 16, change in cystic fibrosis respiratory symptoms through Week 16 as assessed by the CFQ-R respiratory domain score (Table 9), change in weight through Week 16, and rate of pulmonary exacerbation. The overall treatment difference for change from baseline in weight through Week 16 was -0.16 kg (95% CI -1.06, 0.74); the rate ratio for pulmonary exacerbation was 0.677 (95% CI 0.33, 1.37).
| Absolute Change through Week 16 *- Full Analysis Set | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Predicted FEV1
(Percentage Points) | CFQ-R Respiratory Domain Score (Points) | Sweat Chloride (mmol/L) |
||||||||
| Subgroup Parameter | Study Drug | n | Mean | Treatment Difference (95% CI) | n | Mean | Treatment Difference (95% CI) | n | Mean | Treatment Difference (95% CI) |
|
||||||||||
| F508del homozygous | ||||||||||
| Placebo KALYDECO | 28 111 | -0.2 1.5 | 1.72 (-0.6, 4.1) | 28 111 | -1.44 -0.12 | 1.3 (-2.9, 5.6) | 28 109 | 0.13 -2.74 | -2.9 (-5.6, -0.2) |
|
16. How is Kalydeco supplied
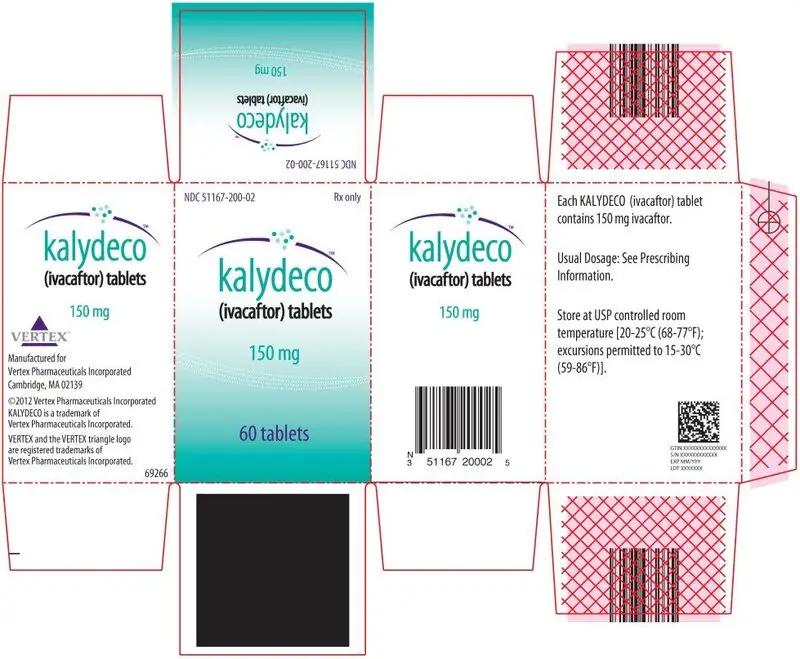
KALYDECO (ivacaftor) tablets are supplied as light blue, film-coated, capsule-shaped tablets containing 150 mg of ivacaftor. Each tablet is printed with the characters "V 150" on one side and plain on the other, and is packaged as follows:
| 56-count carton (contains 4 individual blister cards of 14 tablets per card) | NDC 51167-200-01 | |
| 60-count bottle | NDC 51167-200-02 |
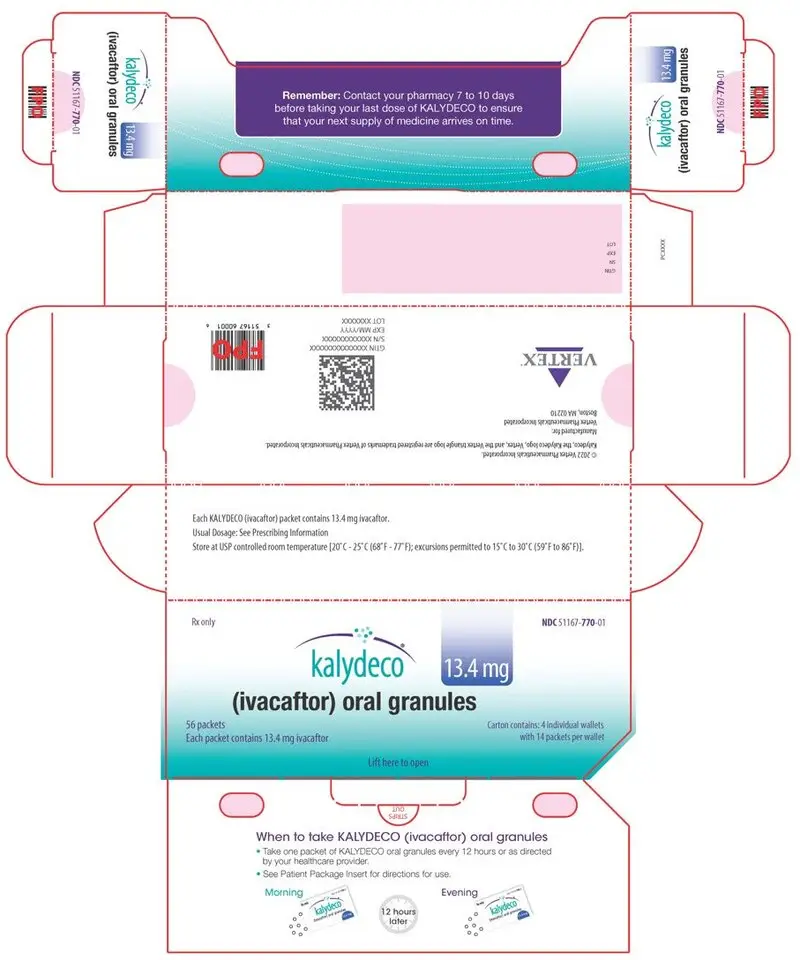
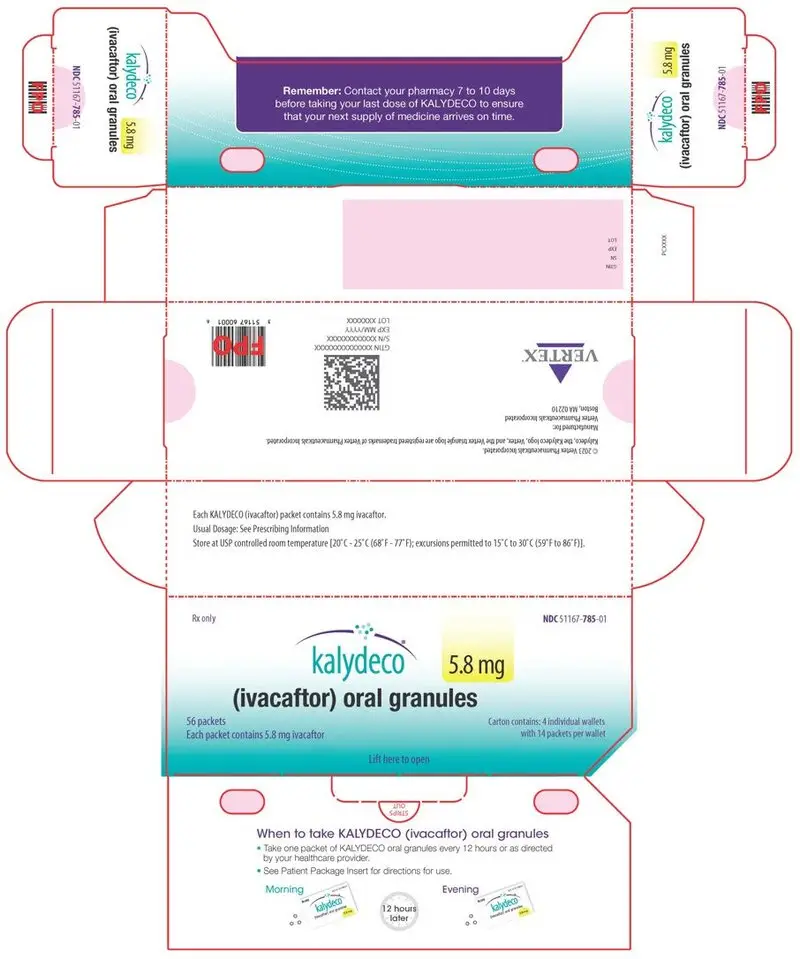
KALYDECO (ivacaftor) oral granules are supplied as small, white to off-white granules and enclosed in unit-dose packets as follows:
| 56-count carton (contains 56 unit-dose packets of 5.8 mg ivacaftor per packet) | NDC 51167-785-01 | |
| 56-count carton (contains 56 unit-dose packets of 13.4 mg ivacaftor per packet) | NDC 51167-770-01 | |
| 56-count carton (contains 56 unit-dose packets of 25 mg ivacaftor per packet) | NDC 51167-600-01 | |
| 56-count carton (contains 56 unit-dose packets of 50 mg ivacaftor per packet) | NDC 51167-300-01 | |
| 56-count carton (contains 56 unit-dose packets of 75 mg ivacaftor per packet) | NDC 51167-400-01 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: 08/2023 | ||
| Patient Information KALYDECO® (kuh-LYE-deh-koh) (ivacaftor) tablets, for oral use (ivacaftor) oral granules |
|||
What is KALYDECO?
|
|||
Before taking KALYDECO, tell your doctor about all of your medical conditions, including if you:
KALYDECO may affect the way other medicines work, and other medicines may affect how KALYDECO works. Ask your doctor or pharmacist for a list of these medicines if you are not sure. Especially tell your doctor if you take:
|
|||
How should I take KALYDECO?
|
|||
What should I avoid while taking KALYDECO?
|
|||
| What are the possible side effects of KALYDECO? KALYDECO can cause serious side effects, including:
|
|||
| The most common side effects of KALYDECO include: | |||
|
|
||
| Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of KALYDECO. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
How should I store KALYDECO?
|
|||
| General information about the safe and effective use of KALYDECO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use KALYDECO for a condition for which it was not prescribed. Do not give KALYDECO to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about KALYDECO that is written for health professionals. |
|||
| What are the ingredients in KALYDECO? Ivacaftor tablets: Active ingredients: ivacaftor. Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The tablet film coat contains: carnauba wax, FD&C Blue #2, PEG 3350, polyvinyl alcohol, talc, and titanium dioxide. The printing ink contains: ammonium hydroxide, iron oxide black, propylene glycol, and shellac. Ivacaftor oral granules: Active ingredients: ivacaftor. Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, mannitol, sucralose, and sodium lauryl sulfate. Manufactured for: Vertex Pharmaceuticals Incorporated, 50 Northern Avenue, Boston, MA 02210 KALYDECO, VERTEX, and the VERTEX triangle logo are registered trademarks of Vertex Pharmaceuticals Incorporated. All other trademarks referenced herein are the property of their respective owners. ©2023 Vertex Pharmaceuticals Incorporated For more information, go to www.kalydeco.com or call 1-877-752-5933. |
|||
| KALYDECO
ivacaftor tablet, film coated |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| KALYDECO
ivacaftor granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| KALYDECO
ivacaftor granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| KALYDECO
ivacaftor granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| KALYDECO
ivacaftor granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| KALYDECO
ivacaftor granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Vertex Pharmaceuticals Incorporated (602478257) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vertex Pharmaceuticals Incorporated | 602478257 | ANALYSIS(51167-200, 51167-300, 51167-400, 51167-600, 51167-770, 51167-785) | |