Drug Detail:Kinevac (Sincalide [ sin-ka-lide ])
Drug Class: Miscellaneous uncategorized agents
Highlights of Prescribing Information
KINEVAC (sincalide for injection), for intravenous use Initial U.S. Approval: 1976
Recent Major Changes
Indications and Usage for Kinevac
Kinevac is a cholecystokinin (CCK) analog indicated in adults to:
- stimulate gallbladder contraction, as may be assessed by various methods of diagnostic imaging, or to obtain by duodenal aspiration a sample of concentrated bile for analysis of cholesterol, bile salts, phospholipids, and crystals. (1)
- stimulate pancreatic secretion in combination with secretin prior to obtaining a duodenal aspirate for analysis of enzyme activity, composition, and cytology. (1)
- accelerate the transit of a barium meal through the small bowel, thereby decreasing the time and extent of radiation associated with fluoroscopy and x-ray examination of the intestinal tract. (1)
Kinevac Dosage and Administration
Recommended Adult Dosage and
Administration by Indication:
To Stimulate Contraction
of the Gallbladder
- 0.02 mcg/kg as a single dose over 30 to 60 seconds via intravenous injection. If satisfactory contraction does not occur in 15 minutes, administer a dose of 0.04 mcg/kg over 30 to 60 seconds. (2.1)
- Alternatively consider an intravenous infusion to reduce gastrointestinal adverse reactions: 0.12 mcg/kg diluted in 100 mL of 0.9% Sodium Chloride Injection USP and infused over 50 minutes at a rate of 2 mL per minute. (2.1, 2.2, 5.3)
To Stimulate Pancreatic Secretion in Combination with Secretin
- 30 minutes after initiation of secretin for injection, administer 0.02 mcg/kg diluted in 30 mL of 0.9% Sodium Chloride Injection USP and infused over 30 minutes at a rate of 1 mL per minute. (2.1, 2.2)
To Accelerate Transit of a Barium Meal Through the Small Intestine
- After the barium meal is beyond the proximal jejunum, administer 0.04 mcg/kg over 30 to 60 seconds via intravenous injection. (2.1)
- If satisfactory transit of the barium meal has not occurred in 30 minutes, administer a second dose of 0.04 mcg/kg over 30 to 60 seconds. (2.1)
- Alternatively consider an intravenous infusion to reduce gastrointestinal adverse reactions: 0.12 mcg/kg diluted in 100 mL 0.9% Sodium Chloride Injection USP and infused over 30 minutes. (2.1, 2.2, 5.3)
Dosage Forms and Strengths
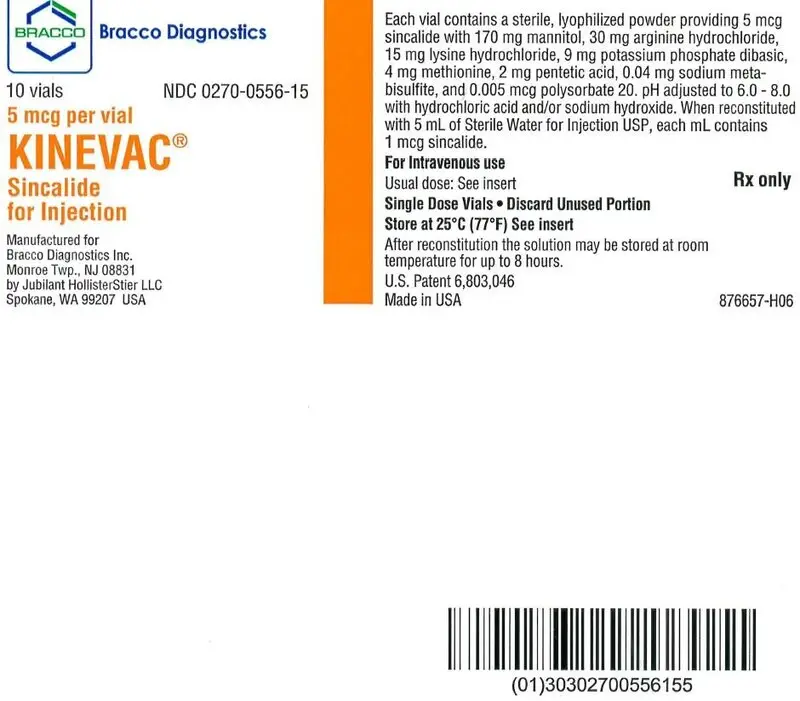
For injection: 5 mcg of sincalide as a lyophilized powder in a single-dose vial for reconstitution (3)
Contraindications
- History of hypersensitivity to sincalide. (4, 5.1)
- Intestinal obstruction. (4)
Warnings and Precautions
- Anaphylaxis, Anaphylactic Shock and Other Hypersensitivity Reactions: May occur during or soon after administration. If symptoms occur, discontinue the drug. (4, 5.1)
- Evacuation of Gallstones: Stimulation of gallbladder contraction in patients with small gallbladder stones could lead to the evacuation of the stones from the gallbladder, resulting in their lodging in the cystic duct or in the common bile duct. (5.2)
- Gastrointestinal Adverse Reactions with Intravenous Injection: Administration as an intravenous injection may cause transient nausea, vomiting, abdominal pain or cramping, dizziness or flushing. To reduce the risk of adverse reactions when used to stimulate contraction of the gallbladder or accelerate transit of a barium meal through the small intestine, administer as an intravenous infusion over 50 or 30 minutes, respectively. (2.1, 5.3)
- Preterm Labor or Spontaneous Abortion: Advise pregnant women of the potential risk for preterm labor and spontaneous abortion. (5.4, 8.1)
Adverse Reactions/Side Effects
Most common adverse reactions (≥20%) are: abdominal discomfort or pain, and nausea. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bracco Diagnostics Inc. at 1-800-257-5181 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Drugs that Affect Gallbladder Motility or Contractile Response: May interfere with response to sincalide. Consider discontinuing these drugs prior to administration of Kinevac, when used to stimulate contraction of the gallbladder. (7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2022
Full Prescribing Information
Indications and Usage for Kinevac
Kinevac is indicated in adults to:
- to stimulate gallbladder contraction, as may be assessed by various methods of diagnostic imaging, or to obtain by duodenal aspiration a sample of concentrated bile for analysis of cholesterol, bile salts, phospholipids, and crystals;
- to stimulate pancreatic secretion in combination with secretin prior to obtaining a duodenal aspirate for analysis of enzyme activity, composition, and cytology;
- to accelerate the transit of a barium meal through the small bowel, thereby decreasing the time and extent of radiation associated with fluoroscopy and x-ray examination of the intestinal tract.
Kinevac Dosage and Administration
2.1 Recommended Dosage and Administration Instructions
Table 1: Recommended Adult Dosage and Administration of Kinevac by Treatment Indication
| Indication | Recommended Adult Dosage and Administration of KINEVAC |
| To stimulate contraction of the gallbladder | |
| To stimulate pancreatic secretion in combination with secretin for injection |
Secretin for Injection: 0.25 units/kg as intravenous infusion over 60 minutes |
| To accelerate the transit of a barium meal through the small intestine |
2.2 Preparation Instructions
- Reconstitute Kinevac aseptically by adding 5 mL of Sterile Water for Injection USP to the vial.
- Inspect the reconstituted solution visually for particulate matter and discoloration after reconstitution and prior to administration.
- Withdraw the prescribed dose of the reconstituted solution from the vial and administer as an intravenous injection over 30 to 60 seconds, as shown in Table 1. Discard the unused portion.
- Store the reconstituted solution at room temperature. Discard after 8 hours.
- For single use only; discard unused portion.
- Reconstitute Kinevac aseptically by adding 5 mL of Sterile Water for Injection USP to the vial.
- After reconstitution, withdraw the prescribed dose of the solution from the vial. Discard unused portion.
- Dilute the reconstituted solution in 30 mL or 100 mL of 0.9% Sodium Chloride Injection USP, depending on the indication, as described in Table 1.
- Inspect the Kinevac solutions visually for particulate matter and discoloration after reconstitution, dilution and prior to administration.
- Store the diluted solution at room temperature. Discard after 1 hour.
Contraindications
KINEVAC is contraindicated in patients with:
- a history of hypersensitivity to sincalide. Serious hypersensitivity reactions have included anaphylaxis and anaphylactic shock [see Warnings and Precautions (5.1), Adverse Reactions (6)].
- intestinal obstruction.
Warnings and Precautions
Adverse Reactions/Side Effects
Less common adverse reactions include:
Neurological reactions: seizures, headache.
Other: nausea, vomiting, flushing, hypertension, urge to defecate, diarrhea, sneezing.
| KINEVAC
sincalide injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bracco Diagnostics Inc. (849234661) |
| Registrant - Bracco Diagnostics Inc. (849234661) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jubilant HollisterStier LLC | 069263643 | ANALYSIS(0270-0556) , MANUFACTURE(0270-0556) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fresenius Kabi USA, LLC | 840771732 | ANALYSIS(0270-0556) , MANUFACTURE(0270-0556) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bachem AG | 482220311 | API MANUFACTURE(0270-0556) | |