Drug Detail:Levora (Ethinyl estradiol and levonorgestrel [ eth-in-ill-ess-tra-dye-ol-and-lee-vo-nor-jess-trel ])
Drug Class: Contraceptives
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs, including LEVORA 0.15/30-28, are contraindicated in women who are over 35 years of age and smoke [see CONTRAINDICATIONS and WARNINGS (1)].
Levora Description
LEVORA® 0.15/30-28 (levonorgestrel and ethinyl estradiol tablets) is a combination oral contraceptive (COC) consisting of 21 white active tablets, each containing 0.15 mg of levonorgestrel, a synthetic progestin and 0.03 mg of ethinyl estradiol, an estrogen, and 7 peach inert tablets (without hormones).
The structural formulas for the active components are:
 |
| Levonorgestrel C21H28O2 MW: 312.4 |
Levonorgestrel is chemically 18,19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-,(17α) (-)-.
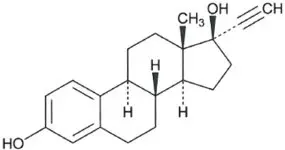 |
| Ethinyl Estradiol C20H24O2 MW: 296.4 |
Ethinyl Estradiol is 19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3, 17-diol.
Each white active tablet contains the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and povidone.
Each peach inert tablet contains the following inactive ingredients: FD&C Yellow No. 6 Lake, Lactose Anhydrous, Lactose Monohydrate, Magnesium Stearate and Microcrystalline Cellulose.
Levora - Clinical Pharmacology
Combination oral contraceptives prevent pregnancy primarily by suppressing ovulation.
Indications and Usage for Levora
LEVORA 0.15/30-28 is indicated for use by females of reproductive potential to prevent pregnancy.
Contraindications
LEVORA 0.15/30-28 is contraindicated in females who are known to have the following conditions:
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
- –
- Smoke, if over age 35 [see BOXED WARNING and WARNINGS (1)].
- –
- Have current or history of deep vein thrombosis or pulmonary embolism [see WARNINGS (1)].
- –
- Have cerebrovascular disease [see WARNINGS (1)].
- –
- Have coronary artery disease [see WARNINGS (1)].
- –
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see WARNINGS (1)].
- –
- Have inherited or acquired hypercoagulopathies [see WARNINGS (1)].
- –
- Have uncontrolled hypertension or hypertension with vascular disease [see WARNINGS (4)].
- –
- Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or vascular disease or other end-organ damage, or diabetes mellitus of >20 years duration [see WARNINGS (8)].
- –
- Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches [see WARNINGS (9)].
- Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive.
- Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis [see WARNINGS (2)].
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see WARNINGS (6)].
- Undiagnosed abnormal uterine bleeding [see WARNINGS (10)].
Warnings
1. Thromboembolic Disorders and Other Vascular Conditions
- Stop LEVORA 0.15/30-28 if an arterial or venous thrombotic/thromboembolic event occurs.
- Stop LEVORA 0.15/30-28 if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.
- Discontinue LEVORA 0.15/30-28 during prolonged immobilization. If feasible, stop LEVORA 0.15/30-28 at least four weeks before and through two weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.
- Start LEVORA 0.15/30-28 no earlier than four weeks after delivery in females who are not breast-feeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the likelihood of ovulation increases after the third postpartum week.
- Before starting LEVORA 0.15/30-28 evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. LEVORA 0.15/30-28 is contraindicated in females with a high risk of arterial or venous thrombotic/thromboembolic diseases (see CONTRAINDICATIONS).
Venous Events
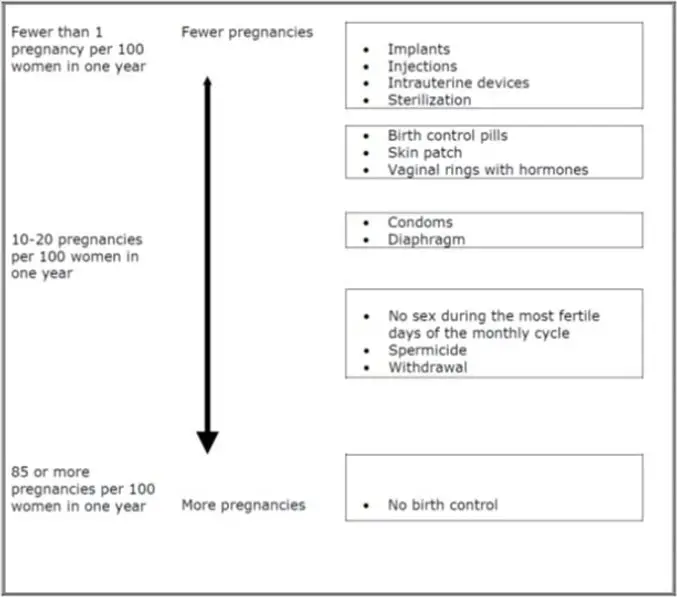
Use of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs (see CONTRAINDICATIONS). While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman-years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, for pregnant females, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Figure 1: Likelihood of Developing a VTE

4. Hypertension
LEVORA 0.15/30-28 is contraindicated in females with uncontrolled hypertension or hypertension with vascular disease (see CONTRAINDICATIONS). For all females, including those with well- controlled hypertension, monitor blood pressure at routine visits and stop LEVORA 0.15/30-28 if blood pressure rises significantly.
An increase in blood pressure has been reported in females using COCs, and this increase is more likely in older women with extended duration of use. The effect of COCs on blood pressure may vary according to the progestin in the COC.
5. Age-related Considerations
The risk for cardiovascular disease and prevalence of risk factors for cardiovascular disease increase with age. Certain conditions, such as smoking and migraine headache without aura, that do not contraindicate COC use in younger females, are contraindications to use in women over 35 years of age [see CONTRAINDICATIONS and WARNINGS (1) ]. Consider the presence of underlying risk factors that may increase the risk of cardiovascular disease or VTE, particularly before initiating a COC for women over 35 years, such as:
- Hypertension
- Diabetes
- Dyslipidemia
- Obesity
6. Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications such as COCs. Discontinue LEVORA 0.15/30-28 prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir (see CONTRAINDICATIONS).
LEVORA 0.15/30-28 can be restarted approximately 2 weeks following completion of treatment with the combination drug regimen.
7. Gallbladder Disease
Studies suggest an increased risk of developing gallbladder disease among COC users. Use of COCs may also worsen existing gallbladder disease.
A past history of COC-related cholestasis predicts an increased risk with subsequent COC use. Females with a history of pregnancy-related cholestasis may be at an increased risk for COC- related cholestasis.
9. Headache
LEVORA 0.15/30-28 is contraindicated in females who have headaches with focal neurological symptoms or have migraine headaches with aura, and in women over age 35 years who have migraine headaches with or without aura (see CONTRAINDICATIONS).
If a woman using LEVORA 0.15/30-28 develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue LEVORA 0.15/30-28 if indicated. Consider discontinuation of LEVORA 0.15/30-28 if there is an increased frequency or severity of migraines during COC use (which may be prodromal of a cerebrovascular event).
11. Depression
Carefully observe females with a history of depression and discontinue LEVORA 0.15/30-28 if depression recurs to a serious degree. Data on the association of COCs with onset of depression or exacerbation of existing depression are limited.
12. Effect on Binding Globulins
The estrogen component of LEVORA 0.15/30-28 may raise the serum concentrations of thyroxine- binding globulin, sex hormone-binding globulin, and cortisol-binding globulin. The dose of replacement thyroid hormone or cortisol therapy may need to be increased.
Precautions
1. Lipid Disorders
Women who are being treated for hyperlipidemias should be followed closely if they elect to use oral contraceptives. Some progestogens may elevate LDL levels and may render the control of hyperlipidemias more difficult [see WARNINGS (8)].
In patients with familial defects of lipoprotein metabolism receiving estrogen-containing preparations, there have been case reports of significant elevations of plasma triglycerides leading to pancreatitis.
2. Fluid Retention
Oral contraceptives may cause some degree of fluid retention. They should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention.
3. Gastrointestinal Motility
Diarrhea and/or vomiting may reduce hormone absorption (see DOSAGE AND ADMINISTRATION).
4. Drug Interactions
The sections below provide information on substances for which data on drug interactions with COCs are available. There is little information available about the clinical effect of most drug interactions that may affect COCs. However, based on the known pharmacokinetic effects of these drugs, clinical strategies to minimize any potential adverse effect on contraceptive effectiveness or safety are suggested.
Consult the approved product labeling of all concurrently used drugs to obtain further information about interactions with COCs or the potential for metabolic enzyme or transporter system alterations.
No drug-drug interaction studies were conducted with LEVORA 0.15/30-28.
4.1 Effects of Other Drugs on Combined Oral Contraceptives
Substances Decreasing the Plasma Concentrations of COCs and Potentially Diminishing the Efficacy of COCs
Table 1 includes substances that demonstrated an important drug interaction with LEVORA 0.15/30-28.
|
|
| Metabolic Enzyme Inducers | |
| Clinical effect |
|
| Prevention or management |
|
| Examples | Aprepitant, barbiturates, bosentan, carbamazepine, efavirenz, felbamate, griseofulvin, oxcarbazepine, phenytoin, rifampin, rifabutin, rufinamide, topiramate, products containing St. John's wort*, and certain protease inhibitors (see separate section on protease inhibitors below). |
| Colesevelam | |
| Clinical effect |
|
| Prevention or management | Administer 4 or more hours apart to attenuate this drug interaction. |
4.2 Effects of Combined Oral Contraceptives on Other Drugs
Table 2 provides significant drug interaction information for drugs co-administered with LEVORA 0.15/30-28.
| Lamotrigine | |
| Clinical effect |
|
| Prevention or management | Dose adjustment may be necessary. Consult the approved product labeling for lamotrigine. |
| Thyroid Hormone Replacement Therapy or Corticosteroid Replacement Therapy | |
| Clinical effect | Concomitant use of COCs with thyroid hormone replacement therapy or corticosteroid replacement therapy may increase systemic exposure of thyroid- binding and cortisol-binding globulin (see Warnings, EFFECT ON BINDING GLOBULINS). |
| Prevention or management | The dose of replacement thyroid hormone or cortisol therapy may need to be increased. Consult the approved product labeling for the therapy in use (see Warnings, EFFECT ON BINDING GLOBULINS). |
| Other Drugs | |
| Clinical effect | Concomitant use of COCs may decrease systemic exposure of acetaminophen, morphine, salicylic acid, and temazepam. Concomitant use with ethinyl estradiol- containing COCs may increase systemic exposure of other drugs (e.g., cyclosporine, prednisolone, theophylline, tizanidine, and voriconazole). |
| Prevention or management | The dosage of drugs that can be affected by this interaction may need to be increased. Consult the approved product labeling for the concomitantly used drug. |
8. Pediatric Use
Safety and efficacy of LEVORA 0.15/30-28 have been established in females of reproductive potential. Use of LEVORA 0.15/30-28 before menarche is not indicated.
9. Geriatric Use
LEVORA 0.15/30-28 has not been studied in postmenopausal women and is not indicated in this population.
10. PATIENT COUNSELING INFORMATION
- Counsel patients that cigarette smoking increases the risk of serious cardiovascular events from COC use, and that women who are over 35 years old and smoke should not use COCs (see BOXED WARNING and CONTRAINDICATIONS).
- Counsel patients that this product does not protect against HIV-infection (AIDS) and other sexually transmitted infections.
- Counsel patients to take one tablet daily by mouth at the same time every day. Instruct patients what to do in the event pills are missed (see DOSAGE AND ADMINISTRATION).
- Counsel patients to use a back-up or alternative method of contraception when enzyme inducers are used with COCs (see [PRECAUTIONS (4.1)].
- Counsel patients who are breastfeeding or who desire to breastfeed that COCs may reduce breast milk production. This is less likely to occur if breastfeeding is well established [see PRECAUTIONS (7)].
- Counsel any patient who starts LEVORA 0.15/30-28 postpartum, and who has not yet had a period, to use an additional method of contraception until she has taken a white tablet for 7 consecutive days (see DOSAGE AND ADMINISTRATION).
- Counsel patients that amenorrhea may occur. Pregnancy should be considered in the event of amenorrhea, and should be ruled out if amenorrhea is associated with symptoms of pregnancy, such as morning sickness or unusual breast tenderness [see WARNINGS (10)]
- Depression may occur. Women should contact their healthcare provider if depression occurs, including shortly after initiating the treatment [see WARNINGS (11)].
Adverse Reactions/Side Effects
Post Marketing Experience
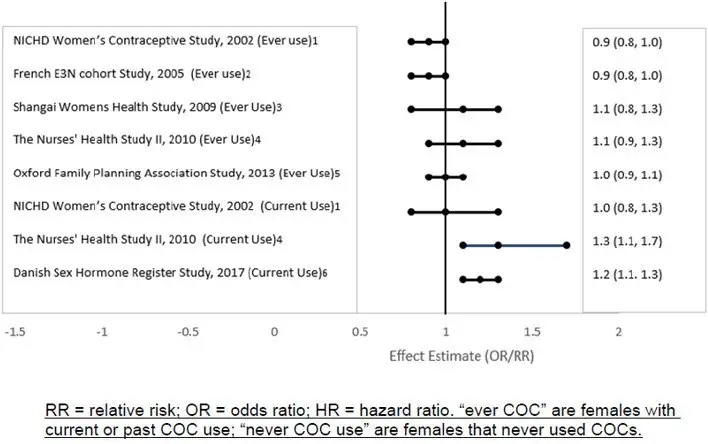
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 - 1.12 (Figure 2).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 2). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 - 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.
Figure 2: Risk of Breast Cancer with Combined Oral Contraceptive Use
The following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling:
- Serious cardiovascular adverse events [see BOXED WARNING and WARNINGS (1)]
- Vascular events [see WARNINGS (1)]
- Liver disease [see WARNINGS (2)]
- Hypertension [see WARNINGS (4)]
- Gallbladder disease [see WARNINGS (7)]
- Carbohydrate and lipid effects [see WARNINGS (8)]
- Headache [see WARNINGS (9)]
- Carcinoma of the cervix [see WARNINGS (3)]
Adverse reactions reported by COC users and described elsewhere in the labeling are:
- Bleeding irregularities and amenorrhea [see WARNINGS (10)]
- Mood changes, including depression [see WARNINGS (11)]
- Melasma/chloasma which may persist [see WARNINGS (14)]
- Edema/fluid retention [see PRECAUTIONS (2)]
- Diminution in lactation when given immediately postpartum [see PRECAUTIONS (7)]
The following adverse reactions have been reported in patients receiving oral contraceptives and are believed to be drug-related: Breast tenderness, pain, enlargement, secretion; Nausea, vomiting and gastrointestinal symptoms (such as abdominal pain, cramps and bloating); Change in menstrual flow; Temporary infertility after discontinuation of treatment; Change in weight or appetite (increase or decrease); Change in cervical erosion and secretion; Cholestatic jaundice; Rash (allergic); Vaginitis, including candidiasis; Change in corneal curvature (steepening); Intolerance to contact lenses; Mesenteric thrombosis; Decrease in serum folate levels; Exacerbation of systemic lupus erythematosus; Exacerbation of porphyria; Exacerbation of chorea; Aggravation of varicose veins; Anaphylactic/anaphylactoid reactions, including urticaria, angioedema, and severe reactions with respiratory and circulatory symptoms.
The following adverse reactions have been reported in users of oral contraceptives, and the association has been neither confirmed nor refuted: Congenital anomalies; Premenstrual syndrome; Cataracts; Optic neuritis, which may lead to partial or complete loss of vision; Cystitis-like syndrome; Nervousness; Dizziness; Hirsutism; Loss of scalp hair; Erythema multiforme; Erythema nodosum; Hemorrhagic eruption; Impaired renal function; Hemolytic uremic syndrome; Budd-Chiari syndrome; Acne; Changes in libido; Colitis; Sickle-cell disease; Cerebral-vascular disease with mitral valve prolapse; Lupus-like syndromes; Pancreatitis; Dysmenorrhea.
Overdosage
There have been no reports of serious adverse outcomes from overdose of COCs, including ingestion by children. Overdose may cause uterine bleeding in females and nausea.
Levora Dosage and Administration
1. How to Start and Take LEVORA 0.15/30-28
LEVORA 0.15/30-28 is dispensed in a compact dispenser containing 28 tablets (see HOW SUPPLIED). LEVORA 0.15/30-28 may be started using either a Day 1 start or a Sunday start (see Table 3). For the first cycle of a Sunday start regimen, an additional method of contraception should be used until after the first 7 consecutive days of administration.
| Starting LEVORA 0.15/30-28 in females with no current use of hormonal contraception | Day 1 start
|
Sunday start
|
|
Switching from another contraceptive method
| Start LEVORA 0.15/30-28 :
|
|
|
|
|
|
|
|
|
|
|
3. Missed doses
Instruct patients about the handling of missed doses (e.g., to take single missed pills as soon as possible) and to follow the dosing instructions provided in the FDA-approved patient labeling.
| Take the tablet as soon as possible. Continue taking one tablet a day until the pack is finished. |
| Take the two missed tablets as soon as possible and the next two active tablets the next day. Continue taking one tablet a day until the pack is finished. Additional nonhormonal contraception (such as condoms or spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. |
| Day 1 start: Throw out the rest of the pack and start a new pack that same day. Sunday start: Continue taking one tablet a day until Sunday, then throw out the rest of the pack and start a new pack that same day. Additional nonhormonal contraception (such as condoms or spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. |
How is Levora supplied
LEVORA® 0.15/30-28 tablets (levonorgestrel and ethinyl estradiol, 0.15 mg/0.03 mg) are available in packages of 6 compact dispensers, each containing 28 tablets:
| 21 Active Tablets: | White, round, unscored, debossed with 15/30 on one side and WATSON on the other side. |
| 7 Inert Tablets: | Peach, round, unscored, debossed with WATSON on one side and P1 on the other side. |
| NDC 51862-097-01 |
Instructions For Use
LEVORA®-0.15/30-28
(levonorgestrel 0.15 mg and ethinyl estradiol tablets 0.03 mg)
Important Information about taking LEVORA 0.15/30-28
- Take 1 pill every day at the same time. Take the pills in the order directed on your pill pack.
- Do not skip your pills, even if you do not have sex often. If you miss pills (including starting the pack late) you could get pregnant. The more pills you miss, the more likely you are to get pregnant.
- If you have trouble remembering to take LEVORA 0.15/30-28, talk to your healthcare provider.
- When you first start taking LEVORA 0.15/30-28, spotting or light bleeding in between your periods may occur. Contact your healthcare provider if this does not go away after a few months.
- You may feel sick to your stomach (nauseous), especially during the first few months of taking LEVORA 0.15/30-28. If you feel sick to your stomach, do not stop taking the pill. The problem will usually go away. If your nausea does not go away, call your healthcare provider.
- Missing pills can also cause spotting or light bleeding, even when you take the missed pills later. On the days you take 2 pills to make up for missed pills (see What should I do if I miss any LEVORA 0.15/30-28 pills? below), you could also feel a little sick to your stomach.
- It is not uncommon to miss a period. However, if you miss a period and have not taken LEVORA 0.15/30-28 according to directions, or feel like you may be pregnant, call your healthcare provider. If you have a positive pregnancy test, you should stop taking LEVORA 0.15/30-28.
- If you have vomiting or diarrhea within 3-4 hours of taking a white pill, take another white pill as soon as possible. Continue taking all your remaining pills in order. Start the first pill of your next pill pack the day after finishing your current pill pack. This will be 1 day earlier than originally scheduled. Continue on your new schedule.
- If you have vomiting or diarrhea for more than 1 day, your birth control pills may not work as well. Use an additional birth control method, like condoms or spermicide, until you check with your healthcare provider.
- Stop taking LEVORA 0.15/30-28 at least 4 weeks before you have major surgery and do not restart after the surgery without asking your healthcare provider. Be sure to use other forms of contraception (like condoms or spermicide) during this time period.
Before you start taking LEVORA 0.15/30-28:
- Decide what time of day you want to take your pill. It is important to take it at the same time every day and in the order as directed on your pill pack.
- Look at your pill pack. Your pill pack consists of 1 card that holds 28 individually sealed pills. The 28 pills consist of 21 white pills (3 rows of 7 pills) and 7 peach pills (1 row of 7 pills). See Figure A.
| Figure A | |
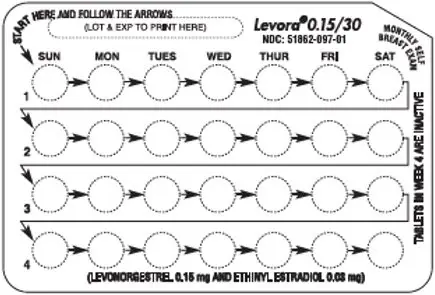 |
- Also find:
- Where on the card to start taking pills (upper left corner) and
- In what order to take the pills (follow the weeks)
- Be sure you have ready at all times another kind of birth control (such as condoms or spermicide), to use as a back-up in case you miss pills.
When should I start taking LEVORA 0.15/30-28?
If you start taking LEVORA 0.15/30-28 and you have not used a hormonal birth control method before:
- There are 2 ways to start taking your birth control pills. You can either start on a Sunday (Sunday Start) or on the first day (Day 1) of your natural menstrual period (Day 1 Start). Your healthcare provider should tell you when to start taking your birth control pill.
- If you use the Sunday Start, use non-hormonal back-up contraception such as condoms or spermicide for the first 7 days that you take LEVORA 0.15/30-28. You do not need back-up contraception if you use the Day 1 Start.
If you start taking LEVORA 0.15/30-28 and you are switching from another birth control pill:
- Start your new LEVORA 0.15/30-28 pack on the same day that you would start the next pack of your previous birth control method.
- Do not continue taking the pills from your previous birth control pill pack.
If you start taking LEVORA 0.15/30-28 and previously used a vaginal ring:
- Start using LEVORA 0.15/30-28 on the day you would have started the next ring.
If you start taking LEVORA 0.15/30-28 and previously used a transdermal patch:
- Start using LEVORA 0.15/30-28 on the day you would have started a new cycle (first patch application).
If you start taking LEVORA 0.15/30-28 and you are switching from a progestin-only method such as an implant or injection:
- Start taking LEVORA 0.15/30-28 on the day of removal of your implant, or on the day when you would have had your next injection.
If you start taking LEVORA 0.15/30-28 and you are switching from an intrauterine device or system (IUD or IUS):
- Start taking LEVORA 0.15/30-28 on the day of removal of your IUD or IUS.
- You do not need back-up contraception if your IUD or IUS is removed on the first day (Day 1) of your period. If your IUD or IUS is removed on any other day, use non-hormonal back-up contraception such as condoms or spermicide for the first 7 days that you take LEVORA 0.15/30-28.
Keep a calendar to track your period: If this is the first time you are taking birth control pills, read, "When should I start taking LEVORA 0.15/30-28?" above. Follow these instructions for either a Sunday Start or a Day 1 Start.
Instructions for using your LEVORA 0.15/30-28 Pill
Dispenser: Sunday Start:
You will use a Sunday Start if your healthcare provider told you to take your first pill on a Sunday.
- Take pill 1 on the Sunday after your period starts. To remove your pill from the dispenser, press the pill through the hole in the bottom of the dispenser.
- If your period starts on a Sunday, take pill "1" that day and refer to Day 1 Start instructions below.
- Take 1 pill every day in the order on the pill dispenser at the same time each day for 28 days.
- After taking the last pill on Day 28 from the pill dispenser, start taking the first pill from a new pack, on the same day of the week as the first pack (Sunday). Take the first pill in the new pack whether or not you are having your period.
- Use non-hormonal back-up contraception such as condoms or spermicide for the first 7 days of the first cycle that you take LEVORA 0.15/30-28.
Day 1 Start:
You will use a Day 1 Start if your doctor told you to take your first pill (Day 1) on the first day of your period.
- Take 1 pill every day in the order of the pill dispenser, at the same time each day, for 28 days. To remove your pill from the dispenser, press the pill through the hole in the bottom of the dispenser.
- After taking the last pill on Day 28 from the pill dispenser, start taking the first pill from a new pack, on the same day of the week as the first pack. Take the first pill in the new pack whether or not you are having your period.
What should I do if I miss any LEVORA 0.15/30-28 pills?
If you miss 1 pill in Weeks 1, 2, or 3, follow these steps:
- Take it as soon as you remember. Take the next pill at your regular time. This means you may take 2 pills in 1 day.
- Then continue taking 1 pill every day until you finish the pack.
- You do not need to use a back-up birth control method if you have sex.
If you miss 2 pills in Week 1 or Week 2 of your pack, follow these steps:
- Take the 2 missed pills as soon as possible and the next 2 pills the next day.
- Then continue to take 1 pill every day until you finish the pack.
- Use a non-hormonal birth control method (such as a condom or spermicide) as a back-up if you have sex during the first 7 days after missing your pills.
If you miss 2 pills in a row in Week 3, or you miss 3 or more pills in a row during Weeks 1, 2, or 3 of the pack, follow these steps:
-
If you are a Day 1 Starter:
- Throw out the rest of the pill pack and start a new pack that same day.
- You may not have your period this month, but this is expected. However, if you miss your period 2 months in a row, call your healthcare provider because you might be pregnant.
- You could become pregnant if you have sex during the first 7 days after you restart your pills. You MUST use a non-hormonal birth control method (such as a condom or spermicide) as a back-up if you have sex during the first 7 days after you restart your pills.
-
If you are a Sunday Starter:
- Keep taking 1 pill every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack of pills that same day.
- Use a non-hormonal birth control method (such as a condom or spermicide) as a back-up if you have sex during the first 7 days after you restart your pills.
If you have any questions or are unsure about the information in this leaflet, call your healthcare provider.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Distributed by:
Mayne Pharma
Greenville, NC 27834
Revised: May 2021
2000013749
| LEVORA
levonorgestrel and ethinyl estradiol kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mayne Pharma Inc. (867220261) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon, Inc. | 240769596 | MANUFACTURE(51862-097) , ANALYSIS(51862-097) , PACK(51862-097) , LABEL(51862-097) | |