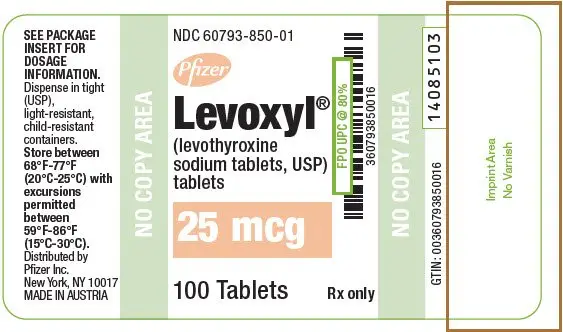
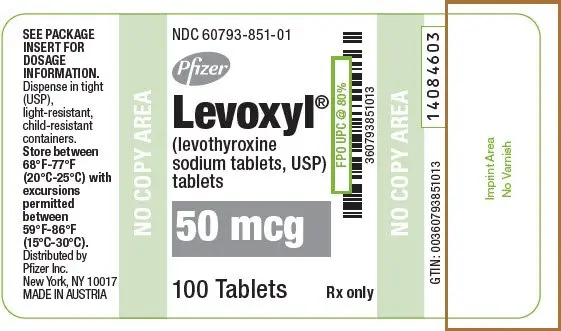
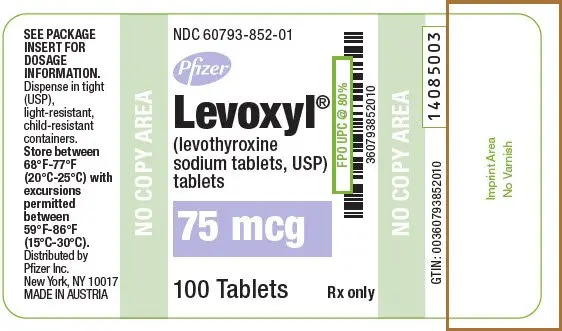
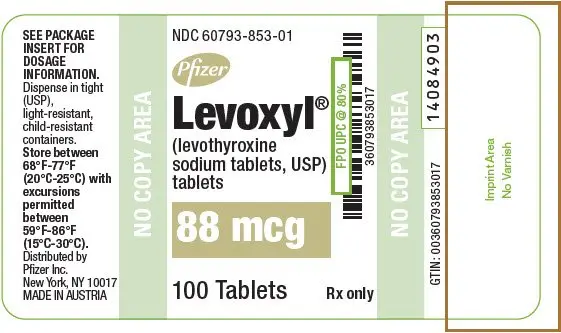
Drug Detail:Levoxyl (Levothyroxine [ lee-voe-thye-rox-een ])
Drug Class: Thyroid drugs
Highlights of Prescribing Information
LEVOXYL® (levothyroxine sodium) tablets, for oral use
Initial U.S. Approval: 2001
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
See full prescribing information for complete boxed warning.
- Thyroid hormones, including LEVOXYL, should not be used for the treatment of obesity or for weight loss.
- Doses beyond the range of daily hormonal requirements may produce serious or even life-threatening manifestations of toxicity (6, 7.7, 10).
Indications and Usage for Levoxyl
LEVOXYL is L-thyroxine (T4) indicated in pediatric and adult patients for:
- Hypothyroidism: As replacement in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism. (1)
- Pituitary Thyrotropin (Thyroid-Stimulating Hormone, TSH) suppression: As an adjunct to surgery and radioiodine therapy in the management of well-differentiated thyroid cancer. (1)
Limitations of Use:
- -
- Not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients. (1)
- -
- Not indicated for treatment of hypothyroidism during the recovery phase of subacute thyroiditis. (1)
Levoxyl Dosage and Administration
- Administer once daily, on an empty stomach, one-half to one hour before breakfast with a full glass of water. (2.1)
- Administer at least 4 hours before or after drugs that are known to interfere with absorption. (2.1)
- Evaluate the need for dose adjustments when regularly administering within one hour of certain foods that may affect absorption. (2.1)
- Starting dose depends on a variety of factors, including age, body weight, cardiovascular status, concomitant medical conditions (including pregnancy), concomitant medications, co-administered food, and the specific nature of the condition being treated. Peak therapeutic effect may not be attained for 4 to 6 weeks. (2.2)
- See full prescribing information for dosing in specific patient populations. (2.3)
- Adequacy of therapy determined with periodic monitoring of TSH and/or T4 as well as clinical status. (2.4)
Dosage Forms and Strengths
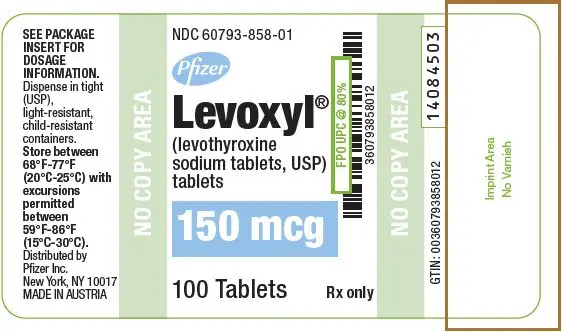
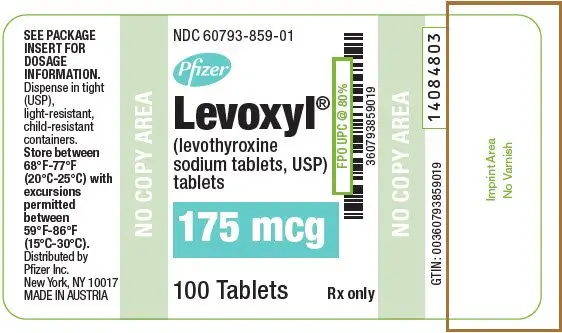
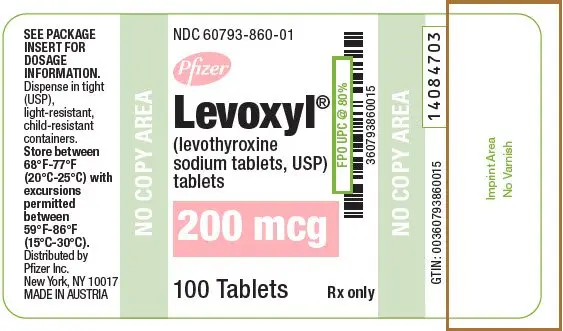
Tablets: 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg (3)
Contraindications
Uncorrected adrenal insufficiency. (4)
Warnings and Precautions
- Cardiac adverse reactions in the elderly and in patients with underlying cardiovascular disease: Initiate LEVOXYL at less than the full replacement dose because of the increased risk of cardiac adverse reactions, including atrial fibrillation. (2.3, 5.1, 8.5)
- Myxedema coma: Do not use oral thyroid hormone drug products to treat myxedema coma. (5.2)
- Acute adrenal crisis in patients with concomitant adrenal insufficiency: Treat with replacement glucocorticoids prior to initiation of LEVOXYL treatment. (5.3)
- Prevention of hyperthyroidism or incomplete treatment of hypothyroidism: Proper dose titration and careful monitoring is critical to prevent the persistence of hypothyroidism or the development of hyperthyroidism. (5.4)
- Worsening of diabetic control: Therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing thyroid hormone therapy. (5.5)
- Decreased bone mineral density associated with thyroid hormone over-replacement: Over-replacement can increase bone resorption and decrease bone mineral density. Give the lowest effective dose. (5.6)
Adverse Reactions/Side Effects
Common adverse reactions for LEVOXYL are primarily those of hyperthyroidism due to therapeutic overdosage: arrhythmias, myocardial infarction, dyspnea, muscle spasm, headache, nervousness, irritability, insomnia, tremors, muscle weakness, increased appetite, weight loss, diarrhea, heat intolerance, menstrual irregularities, and skin rash. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
See full prescribing information for drugs that affect thyroid hormone pharmacokinetics and metabolism (e.g., absorption, synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to LEVOXYL. (7)
Use In Specific Populations
Pregnancy may require the use of higher doses of levothyroxine. (2.3, 8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2018
Full Prescribing Information
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
- Thyroid hormones, including LEVOXYL, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
- In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
- Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects [see Adverse Reactions (6), Drug Interactions (7.7), Overdosage (10)].
1. Indications and Usage for Levoxyl
2. Levoxyl Dosage and Administration
2.1 General Administration Information
Administer LEVOXYL tablets orally as a single daily dose, on an empty stomach, one-half to one hour before breakfast with a full glass of water to avoid choking or gagging [see Adverse Reactions (6)].
Administer LEVOXYL at least 4 hours before or after drugs that are known to interfere with LEVOXYL absorption [see Drug Interactions (7.1)].
Evaluate the need for dose adjustments when regularly administering within one hour of certain foods that may affect LEVOXYL absorption [see Drug Interactions (7.9), Clinical Pharmacology (12.3)].
Administer LEVOXYL to infants and children who cannot swallow intact tablets by crushing the tablet, suspending the freshly crushed tablet in a small amount (5 mL to 10 mL or 1 teaspoon to 2 teaspoons) of water and immediately administering the suspension by spoon or dropper. Do not store the suspension. Do not administer in foods that decrease absorption of LEVOXYL, such as soybean-based infant formula [see Drug Interactions (7.9)].
2.2 General Principles of Dosing
The dose of LEVOXYL for hypothyroidism or pituitary TSH suppression depends on a variety of factors including: the patient's age, body weight, cardiovascular status, concomitant medical conditions (including pregnancy), concomitant medications, co-administered food and the specific nature of the condition being treated [see Dosage and Administration (2.3), Warnings and Precautions (5), Drug Interactions (7)]. Dosing must be individualized to account for these factors and dose adjustments made based on periodic assessment of the patient's clinical response and laboratory parameters [see Dosage and Administration (2.4)].
The peak therapeutic effect of a given dose of LEVOXYL may not be attained for 4 to 6 weeks.
2.3 Dosing in Specific Populations
Pediatric Dosage - Congenital or Acquired Hypothyroidism
The recommended daily dose of LEVOXYL in pediatric patients with hypothyroidism is based on body weight and changes with age as described in Table 1. Start LEVOXYL at the full daily dose in most pediatric patients. Start at a lower starting dose in newborns (0 to 3 months) at risk for cardiac failure and in children at risk for hyperactivity (see below). Monitor for clinical and laboratory response [see Dosage and Administration (2.4)].
| Age | Daily Dose Per Kg Body Weight* |
|---|---|
|
|
| 0 to 3 months | 10 mcg/kg daily to 15 mcg/kg daily |
| 3 to 6 months | 8 mcg/kg daily to 10 mcg/kg daily |
| 6 to 12 months | 6 mcg/kg daily to 8 mcg/kg daily |
| 1 to 5 years | 5 mcg/kg daily to 6 mcg/kg daily |
| 6 to 12 years | 4 mcg/kg daily to 5 mcg/kg daily |
| Greater than 12 years but growth and puberty incomplete | 2 mcg/kg daily to 3 mcg/kg daily |
| Growth and puberty complete | 1.6 mcg/kg daily |
2.4 Monitoring TSH and/or Thyroxine (T4) Levels
Assess the adequacy of therapy by periodic assessment of laboratory tests and clinical evaluation. Persistent clinical and laboratory evidence of hypothyroidism despite an apparent adequate replacement dose of LEVOXYL may be evidence of inadequate absorption, poor compliance, drug interactions, or a combination of these factors.
3. Dosage Forms and Strengths
LEVOXYL tablets are oval, color-coded and, potency marked available as follows:
| Strength (mcg) | Color | Tablet Markings |
|---|---|---|
| 25 | Orange | 25 |
| 50 | White | 50 |
| 75 | Purple | 75 |
| 88 | Olive | 88 |
| 100 | Yellow | 100 |
| 112 | Rose | 112 |
| 125 | Light Brown | 125 |
| 137 | Dark Blue | 137 |
| 150 | Blue | 150 |
| 175 | Turquoise | 175 |
| 200 | Pink | 200 |
4. Contraindications
Levothyroxine is contraindicated in patients with uncorrected adrenal insufficiency [see Warnings and Precautions (5.3)].
5. Warnings and Precautions
5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease
Overtreatment with levothyroxine may cause an increase in heart rate, cardiac wall thickness, and cardiac contractility and may precipitate angina or arrhythmias, particularly in patients with cardiovascular disease and in elderly patients. Initiate LEVOXYL therapy in this population at lower doses than those recommended in younger individuals or in patients without cardiac disease [see Dosage and Administration (2.3), Use in Specific Populations (8.5)].
Monitor for cardiac arrhythmias during surgical procedures in patients with coronary artery disease receiving suppressive LEVOXYL therapy. Monitor patients receiving concomitant LEVOXYL and sympathomimetic agents for signs and symptoms of coronary insufficiency. If cardiovascular symptoms develop or worsen, reduce or withhold the LEVOXYL dose for one week and restart at a lower dose.
5.2 Myxedema Coma
Myxedema coma is a life-threatening emergency characterized by poor circulation and hypometabolism, and may result in unpredictable absorption of levothyroxine sodium from the gastrointestinal tract. Use of oral thyroid hormone drug products is not recommended to treat myxedema coma. Administer thyroid hormone products formulated for intravenous administration to treat myxedema coma.
5.3 Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency
Thyroid hormone increases metabolic clearance of glucocorticoids. Initiation of thyroid hormone therapy prior to initiating glucocorticoid therapy may precipitate an acute adrenal crisis in patients with adrenal insufficiency. Treat patients with adrenal insufficiency with replacement glucocorticoids prior to initiating treatment with LEVOXYL [see Contraindications (4)].
5.4 Prevention of Hyperthyroidism or Incomplete Treatment of Hypothyroidism
LEVOXYL has a narrow therapeutic index. Over- or undertreatment with LEVOXYL may have negative effects on growth and development, cardiovascular function, bone metabolism, reproductive function, cognitive function, emotional state, gastrointestinal function, and on glucose and lipid metabolism. Titrate the dose of LEVOXYL carefully and monitor response to titration to avoid these effects [see Dosage and Administration (2.4)]. Monitor for the presence of drug or food interactions when using LEVOXYL and adjust the dose as necessary [see Drug Interactions (7), Clinical Pharmacology (12.3)].
5.5 Worsening of Diabetic Control
Addition of levothyroxine therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing LEVOXYL [see Drug Interactions (7.2)].
5.6 Decreased Bone Mineral Density Associated with Thyroid Hormone Over-Replacement
Increased bone resorption and decreased bone mineral density may occur as a result of levothyroxine over-replacement, particularly in post-menopausal women. The increased bone resorption may be associated with increased serum levels and urinary excretion of calcium and phosphorous, elevations in bone alkaline phosphatase, and suppressed serum parathyroid hormone levels. Administer the minimum dose of LEVOXYL that achieves the desired clinical and biochemical response to mitigate against this risk.
6. Adverse Reactions/Side Effects
Common adverse reactions with LEVOXYL therapy are primarily those of hyperthyroidism due to therapeutic overdosage [see Warnings and Precautions (5.4), Overdosage (10)]. They include the following:
General: fatigue, increased appetite, weight loss, heat intolerance, fever, excessive sweating
Central nervous system: headache, hyperactivity, nervousness, anxiety, irritability, emotional lability, insomnia
Musculoskeletal: tremors, muscle weakness and cramps
Cardiovascular: palpitations, tachycardia, arrhythmias, increased pulse and blood pressure, heart failure, angina, myocardial infarction, cardiac arrest
Respiratory: dyspnea
Gastrointestinal: diarrhea, vomiting, abdominal cramps, elevations in liver function tests
Dermatologic: hair loss, flushing
Endocrine: decreased bone mineral density
Reproductive: menstrual irregularities, impaired fertility
Seizures have been reported rarely with levothyroxine therapy.
7. Drug Interactions
7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics
Many drugs can exert effects on thyroid hormone pharmacokinetics (e.g., absorption, synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to LEVOXYL (see Tables 2 – 5).
| Potential impact: Concurrent use may reduce the efficacy of LEVOXYL by binding and delaying or preventing absorption, potentially resulting in hypothyroidism. | |
| Drug or Drug Class | Effect |
| Calcium Carbonate Ferrous Sulfate | Calcium carbonate may form an insoluble chelate with levothyroxine, and ferrous sulfate likely forms a ferric-thyroxine complex. Administer LEVOXYL at least 4 hours apart from these agents. |
| Orlistat | Monitor patients treated concomitantly with orlistat and LEVOXYL for changes in thyroid function. |
| Bile Acid Sequestrants -Colesevelam -Cholestyramine -Colestipol Ion Exchange Resins -Kayexalate -Sevelamer | Bile acid sequestrants and ion exchange resins are known to decrease levothyroxine absorption. Administer LEVOXYL at least 4 hours prior to these drugs or monitor thyroid-stimulating hormone (TSH) levels. |
| Other drugs: Proton Pump Inhibitors Sucralfate Antacids - Aluminum & Magnesium Hydroxides - Simethicone | Gastric acidity is an essential requirement for adequate absorption of levothyroxine. Sucralfate, antacids and proton pump inhibitors may cause hypochlorhydria, affect intragastric pH, and reduce levothyroxine absorption. Monitor patients appropriately. |
| Drug or Drug Class | Effect |
|---|---|
| Clofibrate Estrogen-containing Oral Contraceptives Estrogens (oral) Heroin/Methadone 5-Fluorouracil Mitotane Tamoxifen | These drugs may increase serum thyroxine-binding globulin (TBG) concentration. |
| Androgens / Anabolic Steroids Asparaginase Glucocorticoids Slow-Release Nicotinic Acid | These drugs may decrease serum TBG concentration. |
| Potential impact (below): Administration of these agents with LEVOXYL results in an initial transient increase in FT4. Continued administration results in a decrease in serum T4 and normal FT4 and TSH concentrations. | |
| Salicylates (>2 g/day) | Salicylates inhibit binding of T4 and T3 to TBG and transthyretin. An initial increase in serum FT4 is followed by return of FT4 to normal levels with sustained therapeutic serum salicylate concentrations, although total T4 levels may decrease by as much as 30%. |
| Other drugs: Carbamazepine Furosemide (>80 mg IV) Heparin Hydantoins Non-Steroidal Anti-inflammatory Drugs - Fenamates | These drugs may cause protein binding site displacement. Furosemide has been shown to inhibit the protein binding of T4 to TBG and albumin, causing an increased free-T4 fraction in serum. Furosemide competes for T4-binding sites on TBG, prealbumin, and albumin, so that a single high dose can acutely lower the total T4 level. Phenytoin and carbamazepine reduce serum protein binding of levothyroxine, and total and FT4 may be reduced by 20% to 40%, but most patients have normal serum TSH levels and are clinically euthyroid. Closely monitor thyroid hormone parameters. |
| Potential impact: Stimulation of hepatic microsomal drug-metabolizing enzyme activity may cause increased hepatic degradation of levothyroxine, resulting in increased LEVOXYL requirements. | |
| Drug or Drug Class | Effect |
| Phenobarbital Rifampin | Phenobarbital has been shown to reduce the response to thyroxine. Phenobarbital increases L-thyroxine metabolism by inducing uridine 5'-diphospho-glucuronosyltransferase (UGT) and leads to a lower T4 serum levels. Changes in thyroid status may occur if barbiturates are added or withdrawn from patients being treated for hypothyroidism. Rifampin has been shown to accelerate the metabolism of levothyroxine. |
| Potential impact: Administration of these enzyme inhibitors decreases the peripheral conversion of T4 to T3, leading to decreased T3 levels. However, serum T4 levels are usually normal but may occasionally be slightly increased. | |
| Drug or Drug Class | Effect |
| Beta-adrenergic antagonists (e.g., Propranolol >160 mg/day) | In patients treated with large doses of propranolol (>160 mg/day), T3 and T4 levels change slightly, TSH levels remain normal, and patients are clinically euthyroid. It should be noted that actions of particular beta-adrenergic antagonists may be impaired when the hypothyroid patient is converted to the euthyroid state. |
| Glucocorticoids (e.g., Dexamethasone ≥4 mg/day) | Short-term administration of large doses of glucocorticoids may decrease serum T3 concentrations by 30% with minimal change in serum T4 levels. However, long-term glucocorticoid therapy may result in slightly decreased T3 and T4 levels due to decreased TBG production (see above). |
| Other: Amiodarone) | Amiodarone inhibits peripheral conversion of levothyroxine (T4) to triiodothyronine (T3) and may cause isolated biochemical changes (increase in serum free-T4, and decreased or normal free-T3) in clinically euthyroid patients. |
7.2 Antidiabetic Therapy
Addition of LEVOXYL therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control, especially when LEVOXYL is started, changed, or discontinued [see Warnings and Precautions (5.5)].
7.3 Oral Anticoagulants
LEVOXYL increases the response to oral anticoagulant therapy. Therefore, a decrease in the dose of anticoagulant may be warranted with correction of the hypothyroid state or when the LEVOXYL dose is increased. Closely monitor coagulation tests to permit appropriate and timely dosage adjustments.
7.4 Digitalis Glycosides
LEVOXYL may reduce the therapeutic effects of digitalis glycosides. Serum digitalis glycoside levels may be decreased when a hypothyroid patient becomes euthyroid, necessitating an increase in the dose of digitalis glycosides.
7.5 Antidepressant Therapy
Concurrent use of tricyclic (e.g., amitriptyline) or tetracyclic (e.g., maprotiline) antidepressants and LEVOXYL may increase the therapeutic and toxic effects of both drugs, possibly due to increased receptor sensitivity to catecholamines. Toxic effects may include increased risk of cardiac arrhythmias and central nervous system stimulation. LEVOXYL may accelerate the onset of action of tricyclics. Administration of sertraline in patients stabilized on LEVOXYL may result in increased LEVOXYL requirements.
7.6 Ketamine
Concurrent use of ketamine and LEVOXYL may produce marked hypertension and tachycardia. Closely monitor blood pressure and heart rate in these patients.
7.7 Sympathomimetics
Concurrent use of sympathomimetics and LEVOXYL may increase the effects of sympathomimetics or thyroid hormone. Thyroid hormones may increase the risk of coronary insufficiency when sympathomimetic agents are administered to patients with coronary artery disease.
7.8 Tyrosine-Kinase Inhibitors
Concurrent use of tyrosine-kinase inhibitors such as imatinib may cause hypothyroidism. Closely monitor TSH levels in such patients.
7.9 Drug-Food Interactions
Consumption of certain foods may affect LEVOXYL absorption thereby necessitating adjustments in dosing [see Dosage and Administration (2.1)]. Soybean flour (infant formula), cotton seed meal, walnuts, and dietary fiber may bind and decrease the absorption of LEVOXYL from the GI tract. Grapefruit juice may delay the absorption of levothyroxine and reduce its bioavailability.
7.10 Drug-Laboratory Test Interactions
Consider changes in TBG concentration when interpreting T4 and T3 values. Measure and evaluate unbound (free) hormone and/or determine the free T4 index (FT4I) in this circumstance. Pregnancy, infectious hepatitis, estrogens, estrogen-containing oral contraceptives, and acute intermittent porphyria increase TBG concentrations. Nephrosis, severe hypoproteinemia, severe liver disease, acromegaly, androgens, and corticosteroids decrease TBG concentration. Familial hyper- or hypo-thyroxine-binding globulinemias have been described, with the incidence of TBG deficiency approximating 1 in 9000.
8. Use In Specific Populations
8.4 Pediatric Use
The initial dose of LEVOXYL varies with age and body weight. Dosing adjustments are based on an assessment of the individual patient's clinical and laboratory parameters [see Dosage and Administration (2.3, 2.4)].
In children in whom a diagnosis of permanent hypothyroidism has not been established, discontinue LEVOXYL for a trial period, but only after the child is at least 3 years of age. Obtain serum T4 and TSH levels at the end of the trial period, and use laboratory test results and clinical assessments to guide diagnosis and treatment, if warranted.
8.5 Geriatric Use
Because of the increased prevalence of cardiovascular disease among the elderly, initiate LEVOXYL at less than the full replacement dose [see Dosage and Administration (2.3), Warnings and Precautions (5.1)]. Atrial arrhythmias can occur in elderly patients. Atrial fibrillation is the most common of the arrhythmias observed with levothyroxine overtreatment in the elderly.
10. Overdosage
The signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5.4), Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral embolism, shock, coma, and death have been reported. Seizures occurred in a 3-year-old child ingesting 3.6 mg of levothyroxine. Symptoms may not necessarily be evident or may not appear until several days after ingestion of levothyroxine sodium.
Reduce the LEVOXYL dose or temporarily discontinued if signs or symptoms of overdosage occur. Initiate appropriate supportive treatment as dictated by the patient's medical status.
For current information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
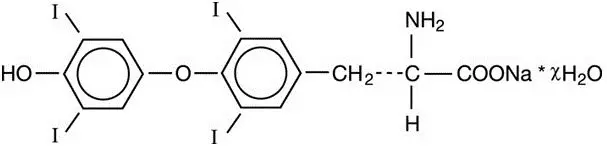
11. Levoxyl Description
LEVOXYL contains the active ingredient, levothyroxine, asynthetic crystalline levothyroxine (T4) in sodium salt form. It is chemically designated as L-3,3',5,5'-tetraiodothyronine monosodium hydrate. Synthetic T4 is identical in chemical structure to the T4 produced in the human thyroid gland. Levothyroxine sodium has an empirical formula of C15H10I4N NaO4 ∙ H2O, molecular weight of 798.85 g/mol (anhydrous), and structural formula as shown:
LEVOXYL tablets for oral administration are supplied in the following strengths: 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, and 200 mcg.
12. Levoxyl - Clinical Pharmacology
12.1 Mechanism of Action
Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and L-thyroxine (T4) diffuse into the cell nucleus and bind to thyroid receptor proteins attached to DNA. This hormone nuclear receptor complex activates gene transcription and synthesis of messenger RNA and cytoplasmic proteins.
The physiological actions of thyroid hormones are produced predominantly by T3, the majority of which (approximately 80%) is derived from T4 by deiodination in peripheral tissues.
12.2 Pharmacodynamics
Oral levothyroxine sodium is a synthetic T4 hormone that exerts the same physiologic effect as endogenous T4, thereby maintaining normal T4 levels when a deficiency is present.
16. How is Levoxyl supplied
LEVOXYL (levothyroxine sodium) tablets are oval, color-coded and, potency marked available as follows:
| Strength (mcg) | Color | Tablet Markings | NDC - bottles of 100 | NDC - bottles of 1000 |
|---|---|---|---|---|
| 25 | Orange | 25 | 60793-850-01 | 60793-850-10 |
| 50 | White | 50 | 60793-851-01 | 60793-851-10 |
| 75 | Purple | 75 | 60793-852-01 | 60793-852-10 |
| 88 | Olive | 88 | 60793-853-01 | 60793-853-10 |
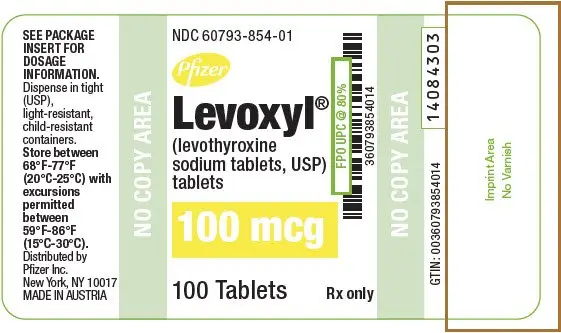
| 100 | Yellow | 100 | 60793-854-01 | 60793-854-10 |
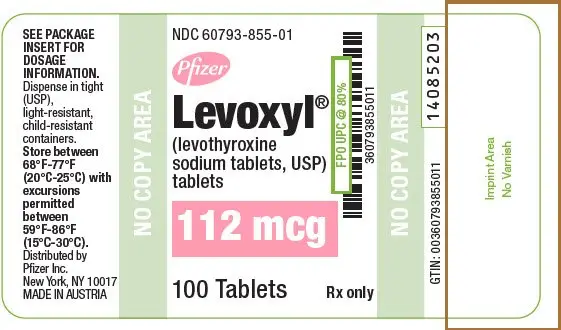
| 112 | Rose | 112 | 60793-855-01 | 60793-855-10 |
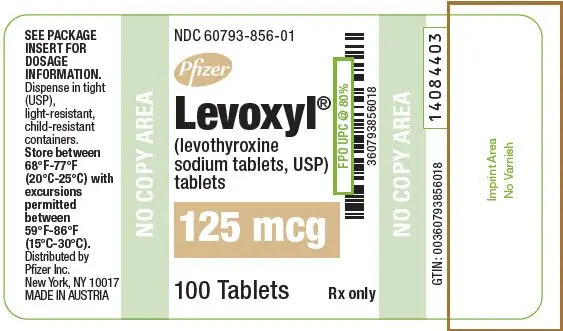
| 125 | Light Brown | 125 | 60793-856-01 | 60793-856-10 |
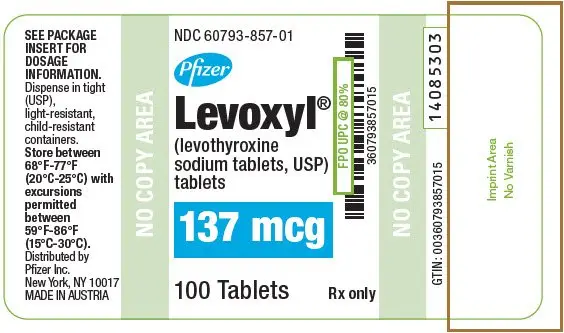
| 137 | Dark Blue | 137 | 60793-857-01 | 60793-857-10 |
| 150 | Blue | 150 | 60793-858-01 | 60793-858-10 |
| 175 | Turquoise | 175 | 60793-859-01 | 60793-859-10 |
| 200 | Pink | 200 | 60793-860-01 | 60793-860-10 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| Patient Information LEVOXYL® (Lev-OX-il) (levothyroxine sodium) tablets, for oral use |
||
|---|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration | Issued:Dec 2018 | |
|
What is the most important information I should know about LEVOXYL?
|
||
| What is LEVOXYL? | ||
LEVOXYL is a prescription medicine that contains a hormone called levothyroxine, which is similar to the hormone produced by your thyroid gland. LEVOXYL is used to treat children and adults:
|
||
| LEVOXYL should not be used to treat people who are recovering from swelling of the thyroid gland (thyroiditis) and whose bodies do not produce enough levothyroxine for a short time. | ||
| Do not use LEVOXYL if your adrenal glands are not working well and you have not been treated for this problem. | ||
Before you take LEVOXYL, tell your healthcare provider about all of your medical conditions, including if you:
|
||
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. LEVOXYL may affect the way other medicines work, and other medicines may affect how LEVOXYL works. You can ask your healthcare provider or pharmacist for a list of medicines that interact with LEVOXYL. | ||
| Tell every healthcare provider including your dentist who treats you that you are taking LEVOXYL before any surgery. | ||
How should I take LEVOXYL?
|
||
Know the medicines that you take. Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
|
||
| What are the possible side effects of LEVOXYL? | ||
LEVOXYL can cause serious side effects, including:
|
||
| The most common side effects of LEVOXYL include: | ||
|
|
|
Other side effects may include:
|
||
| These are not all the possible side effects of LEVOXYL. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
How do I store LEVOXYL?
|
||
| General information about the safe and effective use LEVOXYL. | ||
| Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use LEVOXYL for a condition for which it was not prescribed. Do not give your LEVOXYL to other people, even if they have the same symptoms that you have. It may harm them. | ||
| You can ask your healthcare provider or pharmacist for information about LEVOXYL that was written for health professionals. | ||
| What are the ingredients in LEVOXYL? | ||
| Active ingredient: levothyroxine sodium | ||
| Inactive ingredients: microcrystalline cellulose, croscarmellose sodium, magnesium stearate, calcium sulfate dihydrate, sodium bicarbonate, coloring additives. | ||
| Gluten content: This product is gluten-free. | ||
| This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com. | ||
|
|
||
| LAB-0710-1.0 December 2018 |
||
| For more information, go to www.levoxyl.com, or call 1-866-295-7600 | ||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LEVOXYL
levothyroxine sodium tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |