Drug Detail:Lexette foam (Halobetasol propionate 0.05%)
Drug Class: Topical steroids
Highlights of Prescribing Information
LEXETTE® (halobetasol propionate) topical foam
Initial U.S. Approval: 1990
Recent Major Changes
| Indication and Usage (1) | 05/2021 |
Indications and Usage for Lexette Foam
LEXETTE is a corticosteroid indicated for the topical treatment of plaque psoriasis in patients twelve (12) years of age and older. (1)
Lexette Foam Dosage and Administration
- Shake before use. (2)
- Apply LEXETTE as a thin uniform film to the affected skin twice daily for up to two weeks. Rub in gently. (2)
- Do not use more than 50 grams per week. (2)
- Discontinue LEXETTE when control is achieved. (2)
- If no improvement is seen within 2 weeks, reassess diagnosis. (2)
- Treatment beyond 2 consecutive weeks is not recommended. (2)
- Do not use with occlusive dressings unless directed by a physician. (2)
- Avoid use on the face, groin, or axillae. (2)
- LEXETTE is not for ophthalmic, oral, or intravaginal use. (2)
Dosage Forms and Strengths
Topical foam: 0.05% (0.5 mg/g). (3)
Contraindications
None. (4)
Warnings and Precautions
- Reversible hypothalamic-pituitary-adrenal (HPA) axis suppression may occur, with the potential for glucocorticosteroid insufficiency during or after treatment. Systemic effects following prolonged exposure of topical corticosteroids may also include Cushing's syndrome, hyperglycemia, and glucosuria. Use of potent corticosteroids on large areas, for prolonged durations, under occlusive dressings, or on an altered skin barrier may increase systemic exposure. (5.1)
- Children may be more susceptible to systemic toxicity when treated with topical corticosteroids. (5.1, 8.4)
- Local adverse reactions with topical steroids may include atrophy, striae, irritation, acneiform eruptions, hypopigmentation, and allergic contact dermatitis. Adverse reactions may be more likely to occur with occlusive use or more potent corticosteroids. (5.2)
- Topical corticosteroids may increase the risk of cataract and glaucoma formation. If visual symptoms occur, consider referral to an ophthalmologist for evaluation. (5.3)
- Initiate appropriate therapy if concomitant skin infections develop. (5.4)
- Flammable contents. Avoid heat, flame, or smoking during and immediately following application. (5.6)
Adverse Reactions/Side Effects
The most commonly reported adverse reactions (≥1%) are application site pain and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
Related/similar drugs
Kenalog, Kenalog-40, Dovonex, Trianex, Impoyz, Bryhali, SpevigoFull Prescribing Information
1. Indications and Usage for Lexette Foam
LEXETTE® is indicated for the topical treatment of plaque psoriasis in patients 12 years of age and older.
2. Lexette Foam Dosage and Administration
Shake can prior to use. Apply LEXETTE as a thin uniform film to the affected skin twice daily for up to two weeks. Rub in gently. Wash hands after applying the product.
Discontinue therapy when control is achieved. If no improvement is seen within two weeks, reassessment of the diagnosis may be necessary.
Treatment beyond two weeks is not recommended and the total dosage should not exceed 50 grams per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis [see Warnings and Precautions (5.1)]. Do not use with occlusive dressings unless directed by a physician.
Avoid use on the face, groin, or axillae.
Avoid contact with eyes.
LEXETTE is for topical use only.
LEXETTE is not for ophthalmic, oral, or intravaginal use.
3. Dosage Forms and Strengths
LEXETTE® (halobetasol propionate) topical foam is a white to off-white topical foam. Each gram of LEXETTE, 0.05% contains 0.5 mg of halobetasol propionate.
5. Warnings and Precautions
5.1 Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression and Other Adverse Endocrine Effects
LEXETTE is a topical corticosteroid that has been shown to suppress the hypothalamic-pituitary-adrenal (HPA) axis.
Systemic effects of topical corticosteroids may include reversible HPA axis suppression, with the potential for glucocorticosteroid insufficiency. This may occur during treatment or upon withdrawal of treatment of the topical corticosteroid. The potential for hypothalamic-pituitary adrenal (HPA) suppression with LEXETTE was evaluated in the following studies:
- In a study of 25 adult subjects with moderate to severe plaque psoriasis involving ≥15% of their body surface area. LEXETTE produced laboratory evidence of HPA axis suppression when used twice daily for two weeks in 6 out of 25 (24%) adult subjects with plaque psoriasis. All subjects returned to normal HPA axis function at follow-up at least 4 weeks after stopping the treatment [see Clinical Pharmacology (12.2)].
- In another clinical study, 24 subjects 12 to less than 18 years old with stable plaque psoriasis involving 10% or more of their body surface area applied LEXETTE to affected areas twice daily for two weeks. Of the 23 subjects evaluated for HPA axis suppression, laboratory evidence of adrenal suppression occurred in 6 subjects (26.1%), whom recovered upon retesting after at least 4 weeks of stopping the treatment [see Clinical Pharmacology (12.2)].
Because of the potential for systemic absorption, use of topical corticosteroids, including LEXETTE, may require that patients be evaluated periodically for evidence of HPA axis suppression. Factors that predispose a patient using a topical corticosteroid to HPA axis suppression include the use of more potent corticosteroids, use over large surface areas, prolonged use, occlusive use, use on an altered skin barrier, concomitant use of multiple corticosteroid-containing products, liver failure, and young age. An ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression.
If HPA axis suppression is documented, attempt to gradually withdraw the drug, reduce the frequency of application, or substitute a less potent steroid. Manifestations of adrenal insufficiency may require supplemental systemic corticosteroids. Recovery of HPA axis function is generally prompt and complete upon discontinuation of topical corticosteroids.
Systemic effects of topical corticosteroids may also include Cushing's syndrome, hyperglycemia, and glucosuria. Use of more than one corticosteroid-containing product at the same time may increase the total systemic exposure to topical corticosteroids.
Pediatric patients may be more susceptible than adults to systemic toxicity from the use of topical corticosteroids due to their larger surface-to-body mass ratios [see Use in Specific Populations (8.4)].
5.2 Local Adverse Reactions
Local adverse reactions from topical corticosteroids may include atrophy, striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria. These may be more likely to occur with occlusive use, prolonged use, or use of higher potency corticosteroids, including LEXETTE. Some local adverse reactions may be irreversible.
5.3 Ophthalmic Adverse Reactions
Use of topical corticosteroids may increase the risk of posterior subcapsular cataracts and glaucoma. Cataracts and glaucoma have been reported in postmarketing experience with the use of topical corticosteroid products.
Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
5.4 Concomitant Skin Infections
Use an appropriate antimicrobial agent if a skin infection is present or develops. If a favorable response does not occur promptly, discontinue use of LEXETTE until the infection has been adequately treated.
5.5 Allergic Contact Dermatitis
Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation. Consider confirmation of a clinical diagnosis of allergic contact dermatitis by appropriate patch testing. Discontinue LEXETTE if allergic contact dermatitis is established.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the label:
- Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression and Other Adverse Endocrine Effects [see Warnings and Precautions (5.1)]
- Allergic Contact Dermatitis [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In randomized, multicenter, vehicle-controlled clinical trials, 351 adults with plaque psoriasis were treated with LEXETTE twice daily for up to two weeks (up to approximately 50 grams per week). Table 1 presents selected adverse reactions that occurred in at least 1% of subjects.
| HBP Foam N=351 | Vehicle Foam N=353 |
|
|---|---|---|
| Adverse Reaction | % | % |
| Application site burning/stinging | 12% | 15% |
| Application site pain | 1% | <1% |
| Headache | 1% | <1% |
Skin atrophy (n=1) and telangiectasia (n=2) were reported with LEXETTE, but not with vehicle foam.
6.2 Postmarketing Experience
Because the reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following local adverse reactions have been reported with topical corticosteroids: folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, irritation, striae, and miliaria. They may occur more frequently with the use of occlusive dressings and higher potency corticosteroids, such as halobetasol propionate.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of LEXETTE in patients younger than 12 years of age have not been established; therefore, use in children younger than 12 years is not recommended.
The safety and effectiveness of LEXETTE for the treatment of stable plaque psoriasis in subjects 12 to less than 18 years of age is supported by evidence from adequate and well-controlled studies in adults and from one open-label safety study in 24 subjects aged 12 to less than 18 years. Subjects 12 to less than 18 years with stable plaque psoriasis covering a minimum of 10% of the total body surface area at baseline were treated twice daily for 2 weeks with LEXETTE. Hypothalamic-pituitary adrenal (HPA) axis function (ACTH stimulation test) was evaluated in a subset of 23 subjects. After 2 weeks of treatment, 6 of 23 subjects (26.1%) experienced laboratory evidence of adrenal suppression (i.e., cortisol serum level of ≤18 µg/dL) that recovered upon retesting after at least 4 weeks of stopping the treatment [see Clinical Pharmacology (12.2)].
Because of higher skin surface area to body mass ratios, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing's syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during or after withdrawal of treatment. Adverse reactions including striae have been reported with use of topical corticosteroids in infants and children [see Warnings and Precautions (5.1)].
HPA axis suppression, Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema [see Warnings and Precautions (5.1)].
10. Overdosage
Topically applied LEXETTE can be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5.1)].
11. Lexette Foam Description
LEXETTE is a hydroethanolic aerosol foam that contains a corticosteroid, halobetasol propionate intended for topical use. The chemical name of halobetasol propionate is 21-chloro-6α, 9-difluoro-11β, 17-dihydroxy-16β-methylpregna-1, 4-diene-3,20-dione 17-propionate. Halobetasol propionate is a white to off-white crystalline powder with a molecular weight of 484.96 and a molecular formula of C25H31ClF2O5. It has the following structural formula:
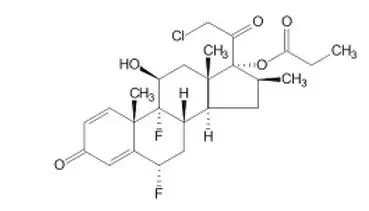
It is practically insoluble in water and freely soluble in dichloromethane and in acetone. Each gram of LEXETTE contains 0.5 mg of halobetasol propionate in a white to off-white foam base consisting of alcohol (specially denatured alcohol [SDA]), benzoic acid, cetostearyl alcohol, emulsifying wax, polyoxyl 20 cetostearyl ether, propylene glycol and purified water. LEXETTE is dispensed from an aluminum can pressurized with a hydrocarbon (isobutane and propane) propellant.
12. Lexette Foam - Clinical Pharmacology
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action in plaque psoriasis is unknown.
12.3 Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption.
In the HPA-axis and pharmacokinetic study, as described above in Clinical Pharmacology (12.2), pharmacokinetics was evaluated in a subgroup of 23 adult subjects with moderate to severe plaque psoriasis following twice daily treatment for 14 days. Plasma concentration of halobetasol propionate (HBP) was measurable in all subjects and steady state was achieved by Day 14. The mean (± standard deviation) Cmax concentration for HBP on Day 14 was 199.7 ± 217.3 pg/mL, with the corresponding median Tmax value of 1 hour (range 0 – 12 hours); mean area under the halobetasol propionate concentration versus time curve over the dosing interval (AUCt) was 1434.9 ± 1310.6 pg∙h/mL.
In the HPA axis study in subjects 12 to less than 18 years [see Clinical Pharmacology (12.2)], trough plasma concentrations of HBP were measured on Day 8 and Day 15 in 23 subjects following twice daily treatment for 14 days. On Day 8, nine (9) of the 23 evaluable subjects had morning trough concentrations of halobetasol propionate in plasma that were above the quantifiable limit (≥20.0 pg/mL); mean halobetasol concentration was 154.6 ± 308.67 pg/mL. Similarly, on Day 15, nine (9) of the 23 evaluable subjects had morning trough concentrations of halobetasol propionate above the quantifiable limit; mean halobetasol concentration was 59.9 ± 90.15 pg/mL. Of the 9 subjects with quantifiable plasma concentrations at Day 15, seven (7) also had quantifiable plasma concentrations at Day 8.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of halobetasol propionate.
In a 90-day repeat-dose toxicity study in rats, topical administration of LEXETTE at dose concentrations from 0.005% to 0.05% or from 0.011 to 0.11 mg/kg/day of halobetasol propionate resulted in a toxicity profile consistent with long-term exposure to corticosteroids including adrenal atrophy, histopathological changes in several organ systems indicative of severe immune suppression, and opportunistic fungal and bacterial infections. A no observable adverse effect level could not be determined in this study. Although the clinical relevance of the findings in animals to humans is not clear, sustained glucocorticoid-related immune suppression may increase the risk of infection and possibly the risk of carcinogenesis.
Halobetasol propionate was not found to be genotoxic in the Ames/Salmonella assay, in the Chinese hamster CHO/HGPRT assay, in the mouse micronucleus test, in the sister chromatid exchange test in somatic cells of the Chinese hamster, or in the chromosome aberration test in somatic cells of Chinese hamsters. Positive mutagenicity effects were observed in two genotoxicity assays: Chinese hamster nuclear anomaly test and mouse lymphoma gene mutation assay in vitro.
Studies in the rat following oral administration at dose levels up to 0.05 mg/kg/day indicated no impairment of fertility or general reproductive performance.
14. Clinical Studies
LEXETTE was evaluated for the treatment of moderate to severe plaque psoriasis in two multicenter, randomized, double-blind, vehicle-controlled studies (Study 1 [NCT02368210] and Study 2 [NCT02742441]).
These studies were conducted in 560 subjects 18 years of age and older with plaque psoriasis involving between 2% and 12% body surface area. Baseline disease severity was determined using a static, five-level Investigator's Global Assessment (IGA) scale, on which a subject scored either moderate or severe. Overall, approximately 60% of subjects were male and approximately 90% were Caucasian.
Subjects applied LEXETTE or vehicle to all affected areas twice daily for up to 14 consecutive days.
The primary measure of efficacy was Overall Treatment Success, defined as the proportion of subjects who were cleared or almost cleared with at least a two-grade improvement from baseline at Week 2 (end of treatment) based on the IGA. The studies also evaluated treatment success for the individual signs of psoriasis (plaque elevation, scaling, and erythema) at the end of treatment. Table 2 presents these results.
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| LEXETTE N=75 | Vehicle Foam N=76 | LEXETTE N=205 | Vehicle Foam N=204 |
|
|
||||
| Overall Treatment Success* | 19 (25%) | 3 (4%) | 63 (31%) | 15 (7%) |
| Plaque Elevation† | 20/75 (27%) | 3/76 (4%) | 71/202 (35%) | 20/203 (10%) |
| Scaling† | 21/75 (28%) | 4/76 (5%) | 68/201 (34%) | 20/204 (10%) |
| Erythema† | 16/75 (21%) | 2/76 (3%) | 59/205 (29%) | 17/204 (8%) |
16. How is Lexette Foam supplied
16.1 How Supplied
LEXETTE, 0.05% is a white to off-white foam. It is supplied in aluminum cans of:
50 grams (NDC 51862-604-50)
100 grams (2 cans of 50 grams) (NDC 51862-604-02)
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all administration instructions or all possible adverse or unintended effects.
Inform patients of the following:
Important Administration Instructions:
- Total dosage should not exceed 50 grams (one can) per week [see Dosage and Administration (2)].
- Advise patients to avoid use on the face, groin, or axillae. Avoid contact with eyes [see Dosage and Administration (2)].
- Inform patients that topical corticosteroids may cause HPA axis suppression and local adverse reactions [see Warnings and Precautions (5.1)].
- Breastfeeding women should not apply LEXETTE directly to the nipple and/or areola to avoid direct exposure to the infant [see Use in Specific Populations (8.2)].
- This product is flammable; avoid heat, flame, or smoking during and immediately following application of this product.
INSTRUCTIONS FOR USELEXETTE® (lex-et)(halobetasol propionate) Topical Foam, 0.05%
Read the Patient Information and Instructions for Use before you use LEXETTE.
Important information: LEXETTE is for skin use only. Do not get LEXETTE in your mouth, eyes, or vagina.
Parts of the LEXETTE can:
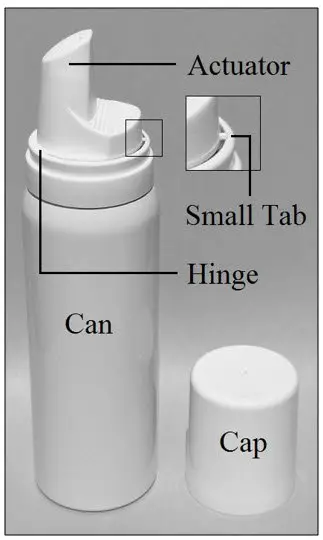
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. | |
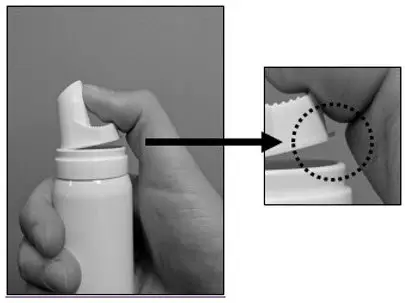
| Step 1: Before applying LEXETTE for the first time, remove cap and break the small tab at the base of the actuator by gently pushing the actuator away from the tab as shown. Do not break the hinge on the actuator. |
|
| Step 2: Shake the can well before use. |
|
| Step 3: Turn the can completely upside down. |
|
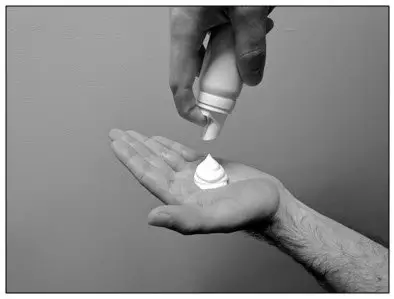
| Step 4: Press down on the actuator to dispense a small amount of the foam into the palm of your hand. |
|
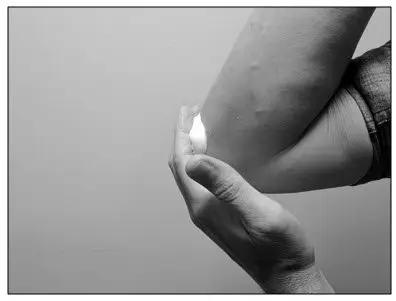
| Step 5: Apply a thin layer of LEXETTE to the affected skin area. Gently rub LEXETTE into the affected skin until the foam disappears. Repeat Steps 4 and 5 to all the affected areas as prescribed by your healthcare provider. |
|
| Step 6: After applying LEXETTE, put the cap back on the can. Step 7: Wash your hands after applying LEXETTE unless you are using the medicine to treat your hands. |
|
Distributed by: Mayne Pharma
Raleigh, NC 27609
U.S. Patent 10,857,159 and 11,020,407
Revised: 03/2023
| LEXETTE
halobetasol propionate aerosol, foam |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Mayne Pharma (867220261) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmasol Corporation | 065144289 | ANALYSIS(51862-604) , MANUFACTURE(51862-604) , PACK(51862-604) , LABEL(51862-604) | |