Drug Detail:Lialda (Mesalamine (oral) [ me-sal-a-meen ])
Drug Class: 5-aminosalicylates
Highlights of Prescribing Information
LIALDA® (mesalamine) delayed-release tablets, for oral use
Initial U.S. Approval: 1987
Recent Major Changes
| Warnings and Precautions, Renal Impairment (5.1) | 11/2022 |
Indications and Usage for Lialda
LIALDA is an aminosalicylate indicated for the:
- induction and maintenance of remission in adult patients with mildly to moderately active ulcerative colitis. (1)
- treatment of mildly to moderately active ulcerative colitis in pediatric patients weighing at least 24 kg. (1)
Lialda Dosage and Administration
Administration Instructions
- Evaluate renal function prior to initiation of LIALDA and periodically while on therapy. (2, 5.1)
- Swallow LIALDA tablets whole; do not split or crush. (2)
- Administer LIALDA tablets with food. (2)
- Drink an adequate amount of fluids. (2, 5.8)
Recommended Dosage in Adults
- For induction of remission: 2.4 g to 4.8 g (two to four 1.2-g tablets) once daily. (2)
- For maintenance of remission: 2.4 g (two 1.2-g tablets) once daily. (2)
Recommended Dosage in Pediatric Patients
- The recommended dosage for treatment of mildly to moderately active ulcerative colitis in pediatric patients weighing at least 24 kg who can swallow tablets whole is shown below: (2)
| Weight of Pediatric Patient | Once Daily LIALDA Dosage | |
|---|---|---|
| Week 0 to Week 8 | After Week 8 | |
| 24 kg to 35 kg | 2.4 g (two 1.2-g tablets) | 1.2 g (one 1.2-g tablet) |
| Greater than 35 kg to 50 kg | 3.6 g (three 1.2-g tablets) | 2.4 g (two 1.2-g tablets) |
| Greater than 50 kg | 4.8 g (four 1.2-g tablets) | 2.4 g (two 1.2-g tablets) |
Dosage Forms and Strengths
Delayed-Release Tablets: 1.2 g (3)
Contraindications
Known or suspected hypersensitivity to salicylates or aminosalicylates or to any of the ingredients of LIALDA. (4)
Warnings and Precautions
- Renal Impairment: Assess renal function at the beginning of treatment and periodically during treatment. Evaluate the risks and benefits of LIALDA in patients with known renal impairment or taking nephrotoxic drugs. Discontinue LIALDA if renal function deteriorates while on therapy. (5.1, 7.1, 8.6)
- Mesalamine-Induced Acute Intolerance Syndrome: Symptoms may be difficult to distinguish from an ulcerative colitis exacerbation. Monitor for worsening symptoms while on treatment. Discontinue treatment if acute intolerance syndrome is suspected. (5.2)
- Hypersensitivity Reactions, including myocarditis and pericarditis: Evaluate patients immediately and discontinue LIALDA if a hypersensitivity reaction is suspected. (5.3)
- Hepatic Failure: Evaluate the risks and benefits of using LIALDA in patients with known liver impairment. (5.4)
- Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. (5.5)
- Upper Gastrointestinal Tract Obstruction: Avoid in patients with pyloric stenosis or other organic or functional obstruction. (5.6)
- Photosensitivity: Advise patients with pre-existing skin conditions to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors. (5.7)
- Nephrolithiasis: Cases of nephrolithiasis have been reported with the use of mesalamine. Mesalamine-containing stones are undetectable by standard radiography or computed tomography (CT). Ensure adequate hydration during treatment. (5.8)
- Interference With Laboratory Tests: Use of mesalamine may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection. (5.9)
Adverse Reactions/Side Effects
Most common adverse reactions in:
- adults (≥2%) are headache, flatulence, liver function test abnormal, abdominal pain, and diarrhea. (6.1)
- pediatric patients (≥5%) are abdominal pain, upper respiratory tract infection, vomiting, anemia, headache, and viral infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals at 1-800-828-2088 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Nephrotoxic Agents including NSAIDs: Increased risk of nephrotoxicity; monitor for changes in renal function and mesalamine-related adverse reactions. (7.1)
- Azathioprine or 6-Mercaptopurine: Increased risk of blood dyscrasias; monitor complete blood cell counts and platelet counts. (7.2)
Use In Specific Populations
Geriatric Patients: Increased risk of blood dyscrasias; monitor complete blood cell counts and platelet counts. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2022
Related/similar drugs
Pentasa, Azulfidine, Apriso, Asacol, Canasa, Delzicol, RowasaFull Prescribing Information
1. Indications and Usage for Lialda
LIALDA is indicated for the:
- induction and maintenance of remission in adult patients with mildly to moderately active ulcerative colitis.
- treatment of mildly to moderately active ulcerative colitis in pediatric patients weighing at least 24 kg.
2. Lialda Dosage and Administration
Pediatric Patients
The recommended dosage for treatment of mildly to moderately active ulcerative colitis in pediatric patients weighing at least 24 kg who can swallow tablets whole is shown in Table 1:
| Weight of Pediatric Patient | Once Daily LIALDA Dosage | |
|---|---|---|
| Week 0 to Week 8 | After Week 8 | |
| 24 kg to 35 kg | 2.4 g (two 1.2-g tablets) | 1.2 g (one 1.2-g tablet) |
| Greater than 35 kg to 50 kg | 3.6 g (three 1.2-g tablets) | 2.4 g (two 1.2-g tablets) |
| Greater than 50 kg | 4.8 g (four 1.2-g tablets) | 2.4 g (two 1.2-g tablets) |
3. Dosage Forms and Strengths
The red-brown ellipsoidal delayed-release tablet containing 1.2 g mesalamine is debossed on one side and imprinted with S476.
4. Contraindications
LIALDA is contraindicated in patients with known or suspected hypersensitivity to salicylates, aminosalicylates, or to any of the ingredients of LIALDA [see Warnings and Precautions (5.3), Adverse Reactions (6.2), Description (11)].
5. Warnings and Precautions
5.1 Renal Impairment
Renal impairment, including minimal change disease, acute and chronic interstitial nephritis, and, rarely, renal failure, has been reported in patients given products such as LIALDA that contain mesalamine or are converted to mesalamine. In animal studies, the kidney was the principal organ of mesalamine toxicity [see Adverse Reactions (6.2), Nonclinical Toxicology (13.2)].
Evaluate renal function prior to initiation of LIALDA therapy and periodically while on therapy. Evaluate the risks and benefits of using LIALDA in patients with known renal impairment, history of renal disease, or taking concomitant nephrotoxic drugs. Discontinue LIALDA if renal function deteriorates while on therapy [see Drug Interactions (7.1), Use in Specific Populations (8.6)].
5.2 Mesalamine-Induced Acute Intolerance Syndrome
Mesalamine has been associated with an acute intolerance syndrome that may be difficult to distinguish from an exacerbation of ulcerative colitis. Although the exact frequency of occurrence has not been determined, it has occurred in 3% of patients in controlled clinical trials of mesalamine or sulfasalazine. Symptoms include cramping, acute abdominal pain, and bloody diarrhea, and sometimes fever, headache, and rash. Monitor patients closely for worsening of these symptoms while on treatment. If acute intolerance syndrome is suspected, promptly discontinue treatment with LIALDA.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions have been reported in patients taking sulfasalazine. Some of these patients may have a similar reaction to LIALDA tablets or to other compounds that contain or are converted to mesalamine.
As with sulfasalazine, mesalamine-induced hypersensitivity reactions may present as internal organ involvement, including myocarditis, pericarditis, nephritis, hepatitis, pneumonitis, and hematologic abnormalities. Evaluate patients immediately if signs or symptoms of a hypersensitivity reaction are present. Discontinue LIALDA if an alternative etiology for the signs or symptoms cannot be established.
5.4 Hepatic Failure
There have been reports of hepatic failure in patients with pre-existing liver disease who have been administered mesalamine. Evaluate the risks and benefits of using LIALDA in patients with known liver impairment.
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported with the use of mesalamine [see Adverse Reactions (6.2)]. Discontinue LIALDA at the first appearance of signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
5.6 Upper Gastrointestinal Tract Obstruction
Pyloric stenosis or other organic or functional obstruction in the upper gastrointestinal tract may cause prolonged gastric retention of LIALDA, which would delay mesalamine release in the colon. Avoid LIALDA in patients at risk of upper gastrointestinal tract obstruction.
5.7 Photosensitivity
Patients with pre-existing skin conditions such as atopic dermatitis and atopic eczema have reported more severe photosensitivity reactions. Advise patients to avoid sun exposure, wear protective clothing, and use a broad-spectrum sunscreen when outdoors.
5.8 Nephrolithiasis
Cases of nephrolithiasis have been reported with the use of mesalamine, including stones with a 100% mesalamine content. Mesalamine-containing stones are radiotransparent and undetectable by standard radiography or computed tomography (CT). Ensure adequate hydration during treatment with LIALDA.
5.9 Interference with Laboratory Tests
Use of LIALDA may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection because of the similarity in the chromatograms of normetanephrine and the main metabolite of mesalamine, N-acetyl-5-aminosalicylic acid (N-Ac-5-ASA). Consider an alternative, selective assay for normetanephrine.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in labeling:
- Renal impairment, including renal failure [see Warnings and Precautions (5.1)]
- Mesalamine-induced acute intolerance syndrome [see Warnings and Precautions (5.2)]
- Hypersensitivity reactions [see Warnings and Precautions (5.3)]
- Hepatic failure [see Warnings and Precautions (5.4)]
- Severe cutaneous adverse reactions [see Warnings and Precautions (5.5)]
- Upper gastrointestinal tract obstruction [see Warnings and Precautions (5.6)]
- Photosensitivity [see Warnings and Precautions (5.7)]
- Nephrolithiasis [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
Induction
The most common adverse reactions occurring in at least 1% of LIALDA- or placebo-treated adult patients with mildly to moderately active ulcerative colitis in two eight-week, randomized, double-blind, placebo-controlled trials (Study 1 and Study 2) [see Clinical Studies (14.1)] are listed in Table 2.
| Adverse Reaction | LIALDA 2.4 g once daily | LIALDA 4.8 g once daily | Placebo |
|---|---|---|---|
| (n=177) | (n=179) | (n=179) | |
|
|||
| Headache | 6% | 3% | <1% |
| Flatulence | 4% | 3% | 3% |
| Liver Function Test Abnormal | <1% | 2% | 1% |
| Alopecia | 0 | 1% | 0 |
| Pruritus | <1% | 1% | 1% |
Pancreatitis occurred in less than 1% of patients during induction in clinical trials and resulted in discontinuation of therapy with LIALDA in patients experiencing this event.
Maintenance of Remission
A LIALDA dosage of 2.4 g/day, administered as either 1.2 g twice daily or 2.4 g once daily, was evaluated for safety in three maintenance trials in patients with mildly to moderately active ulcerative colitis: a 6-month double-blind, active-controlled study (Study 3) [see Clinical Studies (14.1)] and two 12- to 14-month open-label studies. The most common adverse reactions with LIALDA in these maintenance trials are listed in Table 3.
| LIALDA 2.4 g/day† |
|
|---|---|
| (n=1082) | |
| Adverse Reaction | % |
|
|
| Headache | 3% |
| Liver function test abnormal | 2% |
| Abdominal pain | 2% |
| Diarrhea | 2% |
| Abdominal distension | 1% |
| Abdominal pain upper | 1% |
| Dyspepsia | 1% |
| Back pain | 1% |
| Rash | 1% |
| Arthralgia | 1% |
| Fatigue | 1% |
| Hypertension | 1% |
The following adverse reactions, presented by body system, were reported in less than 1% of LIALDA-treated patients with ulcerative colitis in either induction or maintenance trials:
Cardiac Disorder: tachycardia
Ear and Labyrinth Disorders: ear pain
Gastrointestinal Disorders: abdominal distention, colitis, diarrhea, flatulence, nausea, pancreatitis, rectal polyp, vomiting
General Disorders and Administrative Site Disorders: asthenia, face edema, fatigue, pyrexia
Investigations: decreased platelet count
Musculoskeletal and Connective Tissue Disorders: arthralgia, back pain
Nervous System Disorders: dizziness, somnolence, tremor
Respiratory, Thoracic and Mediastinal Disorders: pharyngolaryngeal pain
Skin and Subcutaneous Tissue Disorders: acne, prurigo, rash, alopecia, pruritus, urticaria
Vascular Disorders: hypertension, hypotension
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of LIALDA or other mesalamine-containing products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: lupus-like syndrome, drug fever
Cardiac Disorders: pericarditis, pericardial effusion, myocarditis [see Warnings and Precautions (5.3)]
Gastrointestinal: cholecystitis, gastritis, gastroenteritis, gastrointestinal bleeding, perforated peptic ulcer
Hepatic: jaundice, cholestatic jaundice, hepatitis, liver necrosis, liver failure, hepatotoxicity, Kawasaki-like syndrome including changes in liver enzymes
Hematologic: agranulocytosis, aplastic anemia
Immune System Disorders: anaphylactic reaction, angioedema
Musculoskeletal and Connective Tissue Disorders: myalgia, lupus-like syndrome
Neurological/Psychiatric: peripheral neuropathy, Guillain-Barré syndrome, transverse myelitis, intracranial hypertension
Renal Disorders: renal failure, interstitial nephritis, nephrogenic diabetes insipidus, nephrolithiasis [see Warnings and Precautions (5.1, 5.8)]
Respiratory, Thoracic and Mediastinal Disorders: interstitial lung disease, hypersensitivity pneumonitis (including interstitial pneumonitis, allergic alveolitis, eosinophilic pneumonitis), pleurisy/pleuritis
Skin: psoriasis, pyoderma gangrenosum, erythema nodosum, photosensitivity, SJS/TEN, DRESS, and AGEP [see Warnings and Precautions (5.5)]
Urogenital: reversible oligospermia
7. Drug Interactions
7.1 Nephrotoxic Agents, Including Non-Steroidal Anti-Inflammatory Drugs
The concurrent use of mesalamine with known nephrotoxic agents, including non-steroidal anti-inflammatory drugs (NSAIDs), may increase the risk of nephrotoxicity. Monitor patients taking nephrotoxic drugs for changes in renal function and mesalamine-related adverse reactions [see Warnings and Precautions (5.1)].
7.2 Azathioprine and 6-Mercaptopurine
The concurrent use of mesalamine with azathioprine or 6-mercaptopurine and/or any other drugs known to cause myelotoxicity may increase the risk for blood disorders, bone marrow failure, and associated complications. If concomitant use of LIALDA and azathioprine or 6-mercaptopurine cannot be avoided, monitor blood tests, including complete blood cell counts and platelet counts.
7.3 Interference with Urinary Normetanephrine Measurements
Use of LIALDA may lead to spuriously elevated test results when measuring urinary normetanephrine by liquid chromatography with electrochemical detection [see Warnings and Precautions (5.9)]. Consider an alternative, selective assay for normetanephrine.
8. Use In Specific Populations
8.2 Lactation
Data
In published lactation studies, maternal mesalamine doses from various oral and rectal formulations and products ranged from 500 mg to 4.8 g daily. The average concentration of mesalamine in milk ranged from non-detectable to 0.5 mg/L. The average concentration of N-acetyl-5-aminosalicylic acid in milk ranged from 0.2 to 9.3 mg/L. Based on these concentrations, estimated infant daily dosages for an exclusively breastfed infant are 0 to 0.075 mg/kg/day (RID 0% to 0.1%) of mesalamine and 0.03 to 1.4 mg/kg/day of N-acetyl-5-aminosalicylic acid.
8.4 Pediatric Use
The safety and effectiveness of LIALDA have been established for the treatment of mildly to moderately active ulcerative colitis in pediatric patients weighing at least 24 kg. Use of LIALDA in this population is supported by evidence from adequate and well-controlled trials in adults, a multicenter, randomized, double-blind, parallel group trial in 105 pediatric patients 5 to 17 years of age, and additional pharmacokinetic analyses. The safety profile in pediatric patients was similar to that observed in adults [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.2)].
The safety and effectiveness of LIALDA have not been established in patients weighing less than 24 kg.
8.5 Geriatric Use
Clinical trials of LIALDA did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Reports from uncontrolled clinical studies and postmarketing reporting systems suggested a higher incidence of blood dyscrasias (i.e., agranulocytosis, neutropenia, and pancytopenia) in patients who were 65 years or older who were taking mesalamine-containing products such as LIALDA compared to younger patients. Monitor complete blood cell counts and platelet counts in elderly patients during treatment with LIALDA.
Systemic exposures are increased in elderly subjects [see Clinical Pharmacology (12.3)].
In general, consider the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients when prescribing LIALDA. Consider starting at the low end of the dosing range for induction in elderly patients [see Dosage and Administration (2), Use in Specific Populations (8.6)].
8.6 Renal Impairment
Mesalamine is known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Evaluate renal function in all patients prior to initiation and periodically while on LIALDA therapy. Monitor patients with known renal impairment or history of renal disease or taking nephrotoxic drugs for decreased renal function and mesalamine-related adverse reactions. Discontinue LIALDA if renal function deteriorates while on therapy [see Warnings and Precautions (5.1), Adverse Reactions (6.2), Drug Interactions (7.1)].
10. Overdosage
LIALDA is an aminosalicylate, and symptoms of salicylate toxicity may include nausea, vomiting, abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness, confusion, seizures). Severe intoxication with salicylates may lead to electrolyte and blood pH imbalance, and potentially end organ (e.g., renal and liver) damage.
There is no specific known antidote for mesalamine overdose; however, conventional therapy for salicylate toxicity may be beneficial in the event of acute overdosage and may include gastrointestinal tract decontamination to prevent further absorption. Correct fluid and electrolyte imbalance by the administration of appropriate intravenous therapy and maintain adequate renal function.
LIALDA is a pH-dependent, delayed-release product and this factor should be considered when treating a suspected overdose.
11. Lialda Description
Each LIALDA delayed-release tablet for oral administration contains 1.2 g 5-aminosalicylic acid (5-ASA; mesalamine), an anti-inflammatory agent. Mesalamine also has the chemical name 5-amino-2-hydroxybenzoic acid and its structural formula is:
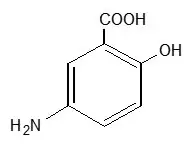 |
|
| Molecular formula: | C7H7NO3 |
| Molecular weight: | 153.14 |
The tablet is coated with a pH-dependent polymer film, which breaks down at or above pH 6.8, normally in the terminal ileum where mesalamine then begins to be released from the tablet core. The tablet core contains mesalamine with hydrophilic and lipophilic excipients and provides for extended release of mesalamine.
The inactive ingredients of LIALDA are sodium carboxymethylcellulose, carnauba wax, stearic acid, silica (colloidal hydrated), sodium starch glycolate (type A), talc, magnesium stearate, methacrylic acid copolymer types A and B, triethylcitrate, titanium dioxide, red ferric oxide, and polyethylene glycol 6000.
12. Lialda - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of mesalamine is not fully understood, but it appears to have a topical anti-inflammatory effect on the colonic epithelial cells. Mucosal production of arachidonic acid metabolites, both through the cyclooxygenase and lipoxygenase pathways, is increased in patients with ulcerative colitis, and it is possible that mesalamine diminishes inflammation by blocking cyclooxygenase and inhibiting prostaglandin production in the colon.
12.3 Pharmacokinetics
Absorption
The total absorption of mesalamine from LIALDA 2.4 g or 4.8 g given once daily for 14 days to healthy subjects was found to be approximately 21% to 22% of the administered dose.
Gamma-scintigraphy studies have shown that a single dose of LIALDA 1.2 g (one tablet) passed intact through the upper gastrointestinal tract of fasted healthy subjects. Scintigraphic images showed a trail of radio-labeled tracer in the colon, suggesting that mesalamine had distributed through this region of the gastrointestinal tract.
In a single-dose study, LIALDA 1.2 g, 2.4 g, and 4.8 g were administered in the fasted state to healthy subjects. Plasma concentrations of mesalamine were detectable after 2 hours and reached a maximum by 9-12 hours on average for the doses studied. The pharmacokinetic parameters are highly variable among subjects (Table 4). Mesalamine systemic exposure in terms of area under the plasma concentration-time curve (AUC) was slightly more than dose proportional between 1.2 g and 4.8 g LIALDA. Maximum plasma concentrations (Cmax) of mesalamine increased approximately dose proportionately between 1.2 g and 2.4 g and sub-proportionately between 2.4 g and 4.8 g of LIALDA, with the dose normalized value at 4.8 g representing, on average, 74% of that at 2.4 g based on geometric means.
| Parameter* of Mesalamine | LIALDA 1.2 g (N=47) | LIALDA 2.4 g (N=48) | LIALDA 4.8 g (N=48) |
|---|---|---|---|
|
|||
| AUC0-t (ng∙h/mL) | 9039† (5054) | 20538 (12980) | 41434 (26640) |
| AUC0-∞ (ng∙h/mL) | 9578‡ (5214) | 21084 (13185) | 44775§ (30302) |
| Cmax (ng/mL) | 857 (638) | 1595 (1484) | 2154 (1140) |
| Tmax¶ (h) | 9.0# (4.0-32.1) | 12.0 (4.0-34.1) | 12.0 (4.0-34.0) |
| Tlag¶ (h) | 2.0# (0-8.0) | 2.0 (1.0-4.0) | 2.0 (1.0-4.0) |
| T1/2 (h) (Terminal Phase) | 8.56‡ (6.38) | 7.05Þ (5.54) | 7.25§ (8.32) |
Specific Populations
Geriatric Patients
In a single-dose pharmacokinetic study of LIALDA, 4.8 g was administered in the fasted state to 71 healthy male and female subjects (28 young (18-35 years); 28 elderly (65-75 years); 15 elderly (>75 years)). Increased age resulted in increased systemic exposure (approximately 2-fold in Cmax) to mesalamine and its metabolite N-acetyl-5-aminosalicylic acid. Increased age resulted in a slower apparent elimination of mesalamine, though there was high between-subject variability.
| Parameter of 5-ASA | Young Subjects (18 to 35 years) (N=28) | Elderly Subjects (65 to 75 years) (N=28) | Elderly Subjects (75 years and older) (N=15) |
|---|---|---|---|
| Arithmetic mean (SD) data are presented, N = Number of subjects; 5-ASA = 5-aminosalicylic acid | |||
|
|||
| AUC0-t (ng∙h/mL) | 51570 (23870) | 73001 (42608) | 65820 (25283) |
| AUC0-∞ (ng∙h/mL) | 58057* (22429) | 89612† (40596) | 63067‡ (22531) |
| Cmax (ng/mL) | 2243 (1410) | 4999 (4381) | 4832 (4383) |
| tmax§ (h) | 22.0 (5.98–48.0) | 12.5 (4.00–36.0) | 16.0 (4.00–26.0) |
| tlag§ (h) | 2 (1–6) | 2 (1–4) | 2 (2–4) |
| t½ (h), terminal phase | 5.68* (2.83) | 9.68† (7.47) | 8.67‡ (5.84) |
| Renal clearance (L/h) | 2.05 (1.33) | 2.04 (1.16) | 2.13 (1.20) |
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
In animal studies with mesalamine, a 13-week oral toxicity study in mice and 13-week and 52-week oral toxicity studies in rats and cynomolgus monkeys have shown the kidney to be the major target organ of mesalamine toxicity. Oral daily doses of 2400 mg/kg in mice and 1150 mg/kg in rats produced renal lesions including granular and hyaline casts, tubular degeneration, tubular dilation, renal infarct, papillary necrosis, tubular necrosis, and interstitial nephritis. In cynomolgus monkeys, oral daily doses of 250 mg/kg or higher produced nephrosis, papillary edema, and interstitial fibrosis.
14. Clinical Studies
14.1 Adults with Mildly to Moderately Active Ulcerative Colitis
Induction of Remission
Two similarly designed, randomized, double-blind, placebo-controlled trials (Study 1, NCT00503243 and Study 2, NCT00548574) were conducted in 517 adult patients with mildly to moderately active ulcerative colitis. The study population was primarily Caucasian (80%), had a mean age of 42 years (6% age 65 years or older), and was approximately 50% male. Both studies used LIALDA dosages of 2.4 g and 4.8 g administered once daily for 8 weeks, except in Study 1 the 2.4 g dosage was administered as two divided doses (i.e., 1.2 g twice daily). The primary efficacy endpoint in both trials was to compare the percentage of patients in remission after 8 weeks of treatment for the LIALDA treatment groups versus placebo. Remission was defined as an Ulcerative Colitis Disease Activity Index (UC-DAI) of ≤1, with scores of zero for rectal bleeding and for stool frequency, and a sigmoidoscopy score reduction of 1 point or more from baseline.
In both studies, the LIALDA dosages of 2.4 g and 4.8 g once daily demonstrated superiority over placebo in the primary efficacy endpoint (Table 6). Both LIALDA dosages also provided consistent benefit in secondary efficacy parameters, including clinical improvement, clinical remission, and sigmoidoscopic improvement. Both LIALDA dosages had similar efficacy profiles.
| Dose | Study 1 (n=262) n/N (%) | Study 2 (n=255) n/N (%) |
|---|---|---|
| LIALDA 2.4 g/day | 30/88 (34) | 34/84 (41) |
| LIALDA 4.8 g/day | 26/89 (29) | 35/85 (41) |
| Placebo | 11/85 (13) | 19/86 (22) |
14.2 Pediatric Patients with Mildly to Moderately Active Ulcerative Colitis Weighing at Least 24 kg
A multicenter, randomized, double-blind, parallel-group study (NCT02093663) was conducted in pediatric patients aged 5 through 17 years with mildly to moderately active ulcerative colitis to determine the safety and effectiveness of LIALDA. The study consisted of two treatment phases, an initial 8-week phase and a 26-week phase. The overall population consisted of 105 patients, of whom 27 patients participated in both the 8-week and 26-week phases.
Each phase included two dosage arms and patients were randomized at the beginning of each phase in a 1:1 ratio, stratified by body weight group. Patients received a low or a high weight-based dosage of LIALDA in four weight groups. Because of the small number of patients in the lowest body weight group (0 in the 8-week phase and 3 in the 26-week phase), the safety and effectiveness of LIALDA in patients weighing less than 24 kg have not been established.
Patients were eligible for the initial 8-week phase if they had mildly to moderately active ulcerative colitis as defined by the UC-DAI score of at least 4 with an endoscopic subscore of 2 or 3.
In the 53 patients enrolled in the initial phase, the mean age and weight of patients was 14 years and 53 kg, the mean (SD) baseline UC-DAI score was 5.8 (1.8), 93% were white, and 59% were male. The primary endpoint was defined by the partial UC-DAI less than or equal to 1 (with rectal bleeding equal to 0, stool frequency less than or equal to 1, and Physician's Global Assessment [PGA] equal to 0). Of the 26 patients in the recommended LIALDA dosage arm, 65% achieved the primary endpoint after 8 weeks of treatment. During the initial 8-week phase, fewer patients who received the recommended LIALDA dosage were discontinued from the study due to ulcerative colitis (0/26, 0%) compared to patients who received a lower than recommended LIALDA dosage (8/27, 30%).
Patients who met the primary endpoint at 8 weeks were eligible to continue treatment in the 26-week phase. Patients were also eligible to enter the 26-week phase without having participated in the 8-week phase if they had a UC-DAI score of less than or equal to 2 with an endoscopic subscore of 0 or 1 (modified to exclude friability).
There were 87 patients enrolled in the 26-week phase. The mean age and weight of patients were 14 years and 54 kg; 97% were white and 55% were female. Of the 42 patients in the recommended LIALDA dosage arm, 55% achieved the primary endpoint, which was defined the same as in the 8-week phase. In the 26-week phase, the arm with a higher than recommended LIALDA dosage was not more effective and is not recommended [see Dosage and Administration (2)].
16. How is Lialda supplied
LIALDA is available as red-brown, ellipsoidal, film-coated, delayed-release tablets containing 1.2 g mesalamine, and debossed on one side imprinted with S476.
NDC 54092-476-12 HDPE Bottle with a child-resistant closure of 120 delayed-release tablets.
| LIALDA
mesalamine tablet, delayed release |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Packaging Services LLC | 002346625 | label(54092-476) , pack(54092-476) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Packaging Services LLC | 143696495 | label(54092-476) , pack(54092-476) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PharmaZell GmbH | 506639652 | api manufacture(54092-476) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cosmo SpA | 630431955 | manufacture(54092-476) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PharmaZell (India) Private Ltd. | 878839106 | api manufacture(54092-476) | |






