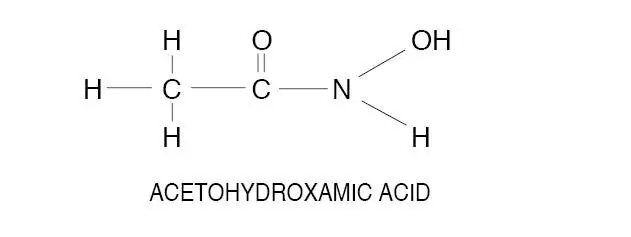
Drug Detail:Lithostat (Acetohydroxamic acid [ a-seet-oh-hye-drox-am-ik-as-id ])
Drug Class: Miscellaneous genitourinary tract agents
Related/similar drugs
amoxicillin, doxycycline, ciprofloxacin, cephalexin, Augmentin, levofloxacin, ceftriaxoneIndications and Usage for Lithostat
Acetohydroxamic acid
should not be used in:
a. patients whose physical state and disease are amenable to definitive surgery and appropriate antimicrobial agents
b. patients whose urine is infected by non-urease producing organisms
c. patients whose urinary infections can be controlled by culture-specific oral antimicrobial agents
d. patients whose renal function is poor (i.e., serum creatinine more than 2.5 mg/dl and/or creatinine clearance less than 20 ml/min)
e. female patients who do not evidence a satisfactory method of contraception
f. patients who are pregnant
Acetohydroxamic acid may cause fetal harm when administered to a pregnant woman. AHA was teratogenic (retarded and/or clubbed rear leg at 750 mg/kg and above and exencephaly and encephalocele at 1,500 mg/kg) when given intraperitoneally to rats.
AHA is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be informed of the potential hazard to the fetus.
Drug Interactions
AHA taken in association with alcoholic beverages has resulted in a rash. (See Adverse Reactions.)
How is Lithostat supplied
LITHOSTAT ®, NDC 0178-0500-01, is available for oral administration as 250 mg white, round tablets, in unit of use packages of 100 tablets. Each LITHOSTAT ® tablet is debossed MPC 500 on one side and blank on the other side. LITHOSTAT ® should be stored in a dry place at room temperature, 15° - 30°C (59° - 86°F). Container should be closed tightly.
L050001R0620
| LITHOSTAT
acetohydroxamic acid tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Mission Pharmacal Company (008117095) |
| Registrant - Mission Pharmacal Company (927726893) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Mission Pharmacal Company | 927726893 | manufacture(0178-0500) | |