Drug Detail:Livmarli (Maralixibat)
Drug Class: Miscellaneous GI agents
Highlights of Prescribing Information
LIVMARLI® (maralixibat) oral solution
Initial U.S. Approval: 2021
Recent Major Changes
| Indications and Usage (1) | 3/2023 |
| Dosage and Administration (2.1, 2.3) | 3/2023 |
Indications and Usage for Livmarli
LIVMARLI is an ileal bile acid transporter (IBAT) inhibitor indicated for the treatment of cholestatic pruritus in patients with Alagille syndrome (ALGS) 3 months of age and older. (1)
Livmarli Dosage and Administration
- The recommended dosage is 380 mcg/kg once daily, taken 30 minutes before a meal in the morning. (2.1)
- Starting dose is 190 mcg/kg orally once daily, and should be increased to 380 mcg/kg once daily after one week, as tolerated. (2.1)
Dosage Forms and Strengths
Oral solution: 9.5 mg of maralixibat per mL. (3)
Contraindications
None. (4)
Warnings and Precautions
- Liver Test Abnormalities: Obtain baseline liver tests and monitor during treatment. Dose reduction or treatment interruption may be considered if abnormalities occur. For persistent or recurrent liver test abnormalities, consider LIVMARLI discontinuation. (5.1)
- Gastrointestinal Adverse Reactions: Consider interrupting LIVMARLI treatment if a patient experiences persistent diarrhea, abdominal pain, vomiting, or has diarrhea with bloody stool, vomiting, dehydration requiring treatment, or fever. If diarrhea, abdominal pain, or vomiting persists and no alternate etiology is identified, consider stopping LIVMARLI treatment. (5.2)
- Fat-Soluble Vitamin (FSV) Deficiency: Obtain baseline levels and monitor during treatment. Supplement if deficiency is observed. If FSV deficiency persists or worsens despite FSV supplementation, consider discontinuing LIVMARLI treatment. (5.3)
Adverse Reactions/Side Effects
Most common adverse reactions (≥5%) are diarrhea, abdominal pain, vomiting, fat-soluble vitamin deficiency, liver test abnormalities, gastrointestinal bleeding, and bone fractures (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Mirum Pharmaceuticals at 1-855-MRM-4YOU or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
Related/similar drugs
Bylvay, odevixibat, maralixibatFull Prescribing Information
1. Indications and Usage for Livmarli
LIVMARLI® is indicated for the treatment of cholestatic pruritus in patients with Alagille syndrome (ALGS) 3 months of age and older.
2. Livmarli Dosage and Administration
2.1 Dosing
The recommended dosage is 380 mcg/kg once daily, taken 30 minutes before a meal in the morning. Start dosing at 190 mcg/kg administered orally once daily; after one week, increase to 380 mcg/kg once daily, as tolerated. The maximum daily dose volume for patients above 70kg is 3 mL or 28.5 mg per day. Refer to the dosing by weight guidelines presented in Table 1.
| Patient Weight (kg) | Days 1-7 (190 mcg/kg once daily) | Beginning Day 8 (380 mcg/kg once daily) |
||
|---|---|---|---|---|
| Volume QD (mL) | Dosing dispenser size (mL) | Volume QD (mL) | Dosing dispenser size (mL) | |
| 5 to 6 | 0.1 | 0.5 | 0.2 | 0.5 |
| 7 to 9 | 0.15 | 0.3 | ||
| 10 to 12 | 0.2 | 0.45 | ||
| 13 to 15 | 0.3 | 0.6 | 1 | |
| 16 to 19 | 0.35 | 0.7 | ||
| 20 to 24 | 0.45 | 0.9 | ||
| 25 to 29 | 0.5 | 1 | ||
| 30 to 34 | 0.6 | 1 | 1.25 | 3 |
| 35 to 39 | 0.7 | 1.5 | ||
| 40 to 49 | 0.9 | 1.75 | ||
| 50 to 59 | 1 | 2.25 | ||
| 60 to 69 | 1.25 | 3 | 2.5 | |
| 70 or higher | 1.5 | 3 | ||
2.2 Missed Dose
If a dose is missed, it should be taken as soon as possible within 12 hours of the time it is usually taken, and the original dosing schedule should be resumed. If a dose is missed by more than 12 hours, the dose can be omitted and the original dosing schedule resumed.
2.3 Important Administration Instructions
Administer LIVMARLI 30 minutes before a meal in the morning [see Clinical Pharmacology (12.3)].
For patients taking bile acid binding resins, take LIVMARLI at least 4 hours before or 4 hours after taking a bile acid binding resin [see Drug Interactions (7.1)].
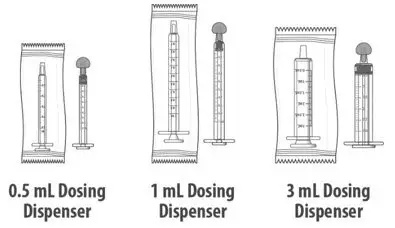
A calibrated measuring device (0.5 mL, 1 mL or 3 mL oral dosing dispenser) will be provided by the pharmacy to measure and deliver the prescribed dose accurately.
After opening the LIVMARLI bottle, store below 30°C (86°F) and discard any remaining LIVMARLI after 100 days.
2.4 Dose Modification for Management of Adverse Events
Establish the baseline pattern of variability of liver tests prior to starting LIVMARLI, so that potential signs of liver injury can be identified. Monitor liver tests (e.g., ALT [alanine aminotransferase], AST [aspartate aminotransferase], TB [total bilirubin]), DB [direct bilirubin] and International Normalized Ratio [INR]) during treatment with LIVMARLI. Interrupt LIVMARLI if new onset liver test abnormalities occur in the absence of other causes. Once the liver test abnormalities either return back to baseline values or stabilize at a new baseline value, consider restarting LIVMARLI at 190 mcg/kg, and increase to 380 mcg/kg as tolerated. Consider discontinuing LIVMARLI permanently if liver test abnormalities recur or symptoms consistent with clinical hepatitis are observed [see Warnings and Precautions (5.1)].
LIVMARLI has not been studied in patients with hepatic decompensation. Discontinue LIVMARLI permanently if a patient experiences a hepatic decompensation event (e.g., variceal hemorrhage, ascites, hepatic encephalopathy).
3. Dosage Forms and Strengths
Oral solution: 9.5 mg of maralixibat per mL as a clear, colorless to yellow solution.
5. Warnings and Precautions
5.1 Liver Test Abnormalities
Patients enrolled in Trial 1 had abnormal liver tests at baseline. During Trial 1, treatment-emergent elevations of liver tests or worsening of liver tests, relative to baseline values, were observed. Most abnormalities included elevation in ALT, AST, or T/DB. In Trial 1, one patient (TB elevated at baseline) discontinued LIVMARLI due to increased TB above baseline after 28 weeks. Four patients had ALT increases that led to dose modification (n=1), dose interruption (n=2), or permanent discontinuation (n=2) of LIVMARLI during the long-term, open-label extension period of Trial 1 [see Adverse Reactions (6.1)].
Obtain baseline liver tests and monitor during treatment. Dose reduction or treatment interruption may be considered if abnormalities occur in the absence of other causes. For persistent or recurrent liver test abnormalities, consider treatment discontinuation.
LIVMARLI was not evaluated in ALGS patients with cirrhosis. Monitor patients during treatment with LIVMARLI for elevations in liver tests and for the development of liver-related adverse reactions. Weigh the potential risks against the benefits of continuing treatment with LIVMARLI in patients who have experienced persistent or recurrent liver tests abnormalities. Discontinue LIVMARLI permanently if a patient progresses to portal hypertension or experiences a hepatic decompensation event.
5.2 Gastrointestinal Adverse Reactions
Diarrhea, abdominal pain, and vomiting were reported as the most common adverse reactions in patients treated with LIVMARLI [see Adverse Reactions (6.1)]. Three patients (3%) experienced vomiting as a serious adverse event requiring hospitalization or intravenous fluid administration.
If diarrhea, abdominal pain, and/or vomiting occur and no other etiologies are found, consider reducing the dose of LIVMARLI or interrupting LIVMARLI dosing. For diarrhea or vomiting, monitor for dehydration and treat promptly. Consider interrupting LIVMARLI dosing if a patient experiences persistent diarrhea or has diarrhea with accompanying signs and symptoms such as bloody stool, vomiting, dehydration requiring treatment, or fever.
When diarrhea, abdominal pain, and/or vomiting resolve, restart LIVMARLI at 190 mcg/kg/day and increase the dose as tolerated. If they recur upon re-challenge with LIVMARLI, then consider stopping LIVMARLI treatment.
5.3 Fat Soluble Vitamin (FSV) Deficiency
Fat-soluble vitamins (FSV) include vitamin A, D, E, and K (measured using INR levels). ALGS patients can have FSV deficiency at baseline. LIVMARLI may affect absorption of fat-soluble vitamins. In Trial 1, treatment emergent FSV deficiency was reported in 3 (10%) patients during 48 weeks of treatment.
Obtain serum FSV levels at baseline and monitor during treatment, along with any clinical manifestations. If FSV deficiency is diagnosed, supplement with FSV. Consider discontinuing LIVMARLI if FSV deficiency persists or worsens despite adequate FSV supplementation.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the Alagille syndrome clinical development program, which includes five clinical studies comprising 86 patients, patients received doses of LIVMARLI up to 760 mcg/kg per day with a median duration of exposure of 32.3 months (range: 0.03 - 60.9 months). In Trial 1, the 4-week placebo control period occurred after 18 weeks of LIVMARLI treatment. In two supportive studies that included long-term open-label extensions, only 13 weeks of placebo-controlled treatment occurred which evaluated doses lower than 380 mcg/kg/day. The majority of LIVMARLI exposure in the development program occurred without a placebo control in open-label trial extensions.
The most common adverse reactions (≥5%) for ALGS patients treated with LIVMARLI are presented in Table 2 below. Treatment interruptions or dose reductions occurred in 5 (6%) patients due to diarrhea, abdominal pain, or vomiting.
| LIVMARLI (n=86) | ||
|---|---|---|
| Adverse Reaction | Any Grade n (%) | Number of events per 100 person-years* |
|
||
| Diarrhea | 48 (55.8%) | 41.6 |
| Abdominal pain† | 46 (53.5%) | 38.6 |
| Vomiting | 35 (40.7%) | 19.8 |
| Nausea | 7 (8.1%) | 2.9 |
| Fat-Soluble Vitamin deficiency† | 22 (25.6%) | 11.1 |
| Transaminases increased (ALT, AST)† | 16 (18.6%) | 6.9 |
| Gastrointestinal Bleeding† | 9 (10.4%) | 3.8 |
| Bone Fractures† | 8 (9.3%) | 3.3 |
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of LIVMARLI for the treatment of cholestatic pruritus in Alagille syndrome have been established in pediatric patients aged 3 months of age and older. Use of LIVMARLI in this population is supported by evidence from a study of patients 1 to 15 years of age (N=31) that included 18 weeks of open-label treatment followed by a 4 week placebo-controlled randomized withdrawal period and a subsequent 26-week open-label treatment period. Additional safety information was obtained from four studies in patients up to 21 years of age (N=55) [see Adverse Reactions (6) and Clinical Studies (14)]. Use of LIVMARLI in patients 3 to <12 months of age is supported by an open-label, multicenter study of LIVMARLI which showed a similar safety, tolerability and pharmacokinetic profile to patients with ALGS ≥12 months of age.
The safety and effectiveness of LIVMARLI have not been established in patients less than 3 months of age.
8.5 Geriatric Use
The safety and effectiveness of LIVMARLI for the treatment of pruritus in ALGS in adult patients, 65 years of age and older, have not been established.
8.7 Hepatic impairment
Clinical studies of LIVMARLI included ALGS patients with impaired hepatic function at baseline. The efficacy and safety in ALGS patients with clinically significant portal hypertension and in patients with decompensated cirrhosis have not been established [see Clinical Studies (14), Dosage and Administration (2.4), and Warnings and Precautions (5.1)].
10. Overdosage
Single doses of maralixibat up to 500 mg, approximately 18-fold higher than the recommended dose, have been administered in healthy adults and were tolerated without a meaningful increase in adverse effects when compared to lower doses. If an overdose occurs, discontinue LIVMARLI, monitor the patient for any signs and symptoms and institute general supportive measures if needed.
LIVMARLI contains propylene glycol (364.5 mg/mL) as an excipient. Oral doses of propylene glycol up to 50 mg/kg/day (1 month to <5 years of age) and 500 mg/kg/day (≥5 years of age) are generally considered safe. Overdoses of propylene glycol may manifest with hyperosmolality, CNS, cardiovascular, and/or respiratory effects and may subside with the elimination of propylene glycol.
11. Livmarli Description
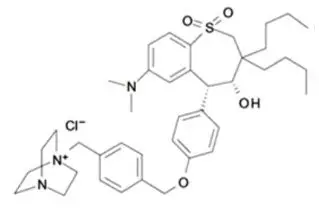
LIVMARLI (maralixibat) oral solution is an ileal bile acid transporter (IBAT) inhibitor. Maralixibat is present as a chloride salt with the chemical name 1-[[4-[[4-[(4R,5R)-3,3-dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl]phenoxy]methyl]phenyl]methyl]-4-aza-1-azoniabicyclo[2.2.2]octane chloride. The molecular formula of maralixibat chloride is C40H56ClN3O4S with a molecular weight of 710.42. It has the following chemical structure:
LIVMARLI is supplied in a multiple-dose bottle containing 9.5 mg of maralixibat per mL (equivalent to 10 mg of maralixibat chloride per mL). The oral solution contains the following inactive ingredients: edetate disodium, grape flavor, propylene glycol, purified water, and sucralose. The pH of the oral solution is 3.8 – 4.8.
12. Livmarli - Clinical Pharmacology
12.1 Mechanism of Action
Maralixibat is a reversible inhibitor of the ileal bile acid transporter (IBAT). It decreases the reabsorption of bile acids (primarily the salt forms) from the terminal ileum.
Pruritus is a common symptom in patients with ALGS and the pathophysiology of pruritus in patients with ALGS is not completely understood. Although the complete mechanism by which maralixibat improves pruritus in ALGS patients is unknown, it may involve inhibition of the IBAT, which results in decreased reuptake of bile salts, as observed by a decrease in serum bile acids [see Clinical Pharmacology (12.2)].
12.2 Pharmacodynamics
In Trial 1, pediatric patients with ALGS were administered open-label treatment with LIVMARLI 380 mcg/kg once daily for 13 weeks after an initial 5-week dose-escalation period [see Clinical Studies (14)]. At baseline, serum bile acids were highly variable among patients ranging from 20 to 749 µmol/L and mean (SD) serum bile acid level was 283 (210.6) µmol/L. Serum bile acid levels decreased from baseline in the majority of patients as early as at Week 12 and the reduction in serum bile acids was generally maintained for the treatment period.
12.3 Pharmacokinetics
Because of the low systemic absorption of maralixibat, pharmacokinetic parameters cannot be reliably calculated at the recommended dose. Concentrations of maralixibat in the pediatric ALGS patients were below the limit of quantification (0.25 ng/mL) in the majority of plasma samples. In Trial 1, the highest concentration of maralixibat in pediatric ALGS patients following treatment with LIVMARLI 380 mcg/kg once daily was 5.93 ng/mL.
Following single oral administration of maralixibat in healthy adults at doses ranging from 1 mg to 500 mg, plasma concentrations of maralixibat were below the limit of quantification (0.25 ng/mL) at doses less than 20 mg and PK parameters could not be reliably estimated.
Following a single dose administration of 30 mg under fasted condition, median Tmax was 0.75 and mean (SD) Cmax and AUClast were 1.65 (1.10) ng/ml and 3.43 (2.13) ng∙h/mL, respectively.
14. Clinical Studies
The efficacy of LIVMARLI was assessed in Trial 1 (NCT02160782), which consisted of an 18-week open-label treatment period; a 4-week randomized, double-blind, placebo-controlled drug-withdrawal period; a subsequent 26-week open-label treatment period; and a long-term open-label extension period.
Thirty-one pediatric ALGS patients with cholestasis and pruritus were enrolled, with 90.3% of patients receiving at least one medication to treat pruritus at study entry. All patients had JAGGED1 mutation. Patients were administered open-label treatment with LIVMARLI 380 mcg/kg once daily for 13 weeks after an initial 5-week dose-escalation period; two patients discontinued treatment during this first 18 weeks of open-label treatment. The 29 patients who completed the open-label treatment phase were then randomized to continue treatment with LIVMARLI or receive matching placebo during the 4-week drug withdrawal period at Weeks 19-22 (n=16 placebo, n=13 LIVMARLI). All 29 patients completed the randomized, blinded drug withdrawal period; subsequently, patients received open-label LIVMARLI at 380 mcg/kg once daily for an additional 26 weeks.
Randomized patients had a median age of 5 years (range: 1 to 15 years) and 66% were male. The baseline mean (standard deviation [SD]) of liver test parameters were as follows: serum bile acid levels 280 (213) µmol/L, AST 158 (68) U/L, ALT 179 (112) U/L, Gamma Glutamyl Transferase (GGT) 498 (399) U/L, and TB 5.6 (5.4) mg/dL.
Given the patients' young age, a single-item observer-reported outcome was used to measure patients' pruritus symptoms as observed by their caregiver twice daily (once in the morning and once in the evening) on the Itch Reported Outcome Instrument (ItchRO[Obs]). Pruritus symptoms were assessed on a 5-point ordinal response scale, with scores ranging from 0 (none observed or reported) to 4 (very severe). Patients were included in Trial 1 if their average pruritus score was greater than 2.0 (moderate) in the 2 weeks prior to baseline.
The average of the worst daily ItchRO(Obs) pruritus scores was computed for each week. For randomized patients, the mean (SD) at baseline (pre-treatment) was 3.1 (0.5) and the mean (SD) at Week 18 (pre-randomized withdrawal period) was 1.4 (0.9). On average, patients administered LIVMARLI for 22 weeks maintained pruritus reduction whereas those in the placebo group who were withdrawn from LIVMARLI after Week 18 returned to baseline pruritus scores by Week 22. Results from the placebo-controlled period are presented in Table 3. After re-entering the open-label treatment phase, both randomized treatment groups had similar mean pruritus scores by Week 28, the first week placebo patients received the full dosage of LIVMARLI after withdrawal. These observer-rated pruritus results are supported by similar results on patient-rated pruritus in patients 5 years of age and older who were able to self-report their itching severity.
| Maralixibat (N=13) | Placebo (N=16) | Mean Difference | |
|---|---|---|---|
| Results based on an analysis of covariance model with treatment group and Week 18 average worst daily pruritus score as covariates | |||
| Week 22, Mean (95% CI) | 1.6 (1.1, 2.1) | 3.0 (2.6, 3.5) | |
| Change from Week 18 to Week 22, Mean (95% CI) | 0.2 (-0.3, 0.7) | 1.6 (1.2, 2.1) | -1.4 (-2.1, -0.8) |
17. Patient Counseling Information
Advise the patient or their caregiver(s) to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
| INSTRUCTIONS FOR USE LIVMARLI® [liv-MAR-lee] (maralixibat) oral solution |
|||
| This Instructions for Use contains information on how to take LIVMARLI. Read this Instructions for Use before you start taking LIVMARLI for the first time and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. | |||
| Follow your healthcare provider's instructions for the dose of LIVMARLI to give. | |||
| Ask your healthcare provider or pharmacist if you have questions about how to prepare or give the prescribed dose of LIVMARLI. | |||
| Important information about measuring LIVMARLI | |||
|
|||
| Table 1 | |||
| Dose (mL) | Dosing dispenser size (mL) | ||
| 0.1 to 0.5 | 0.5 | ||
| 0.6 to 1 | 1 | ||
| 1.25 to 3 | 3 | ||
|
|||
| Storage information | |||
|
|||
| Keep LIVMARLI and all medicines out of the reach of children. | |||
| You will receive | |||
| LIVMARLI oral solution | |||
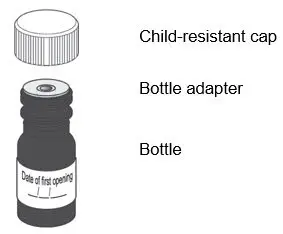
| Each package of LIVMARLI contains: | |||
|
LIVMARLI (9.5 mg/mL):
|
|||
| Dosing dispensers, provided separately by your pharmacist: | |||
|
|
|||
| Note: Dosing dispenser sizes shown are for example only. | |||
| Section A: Prepare the bottle | |||
| 1. Remove the LIVMARLI bottle from the box (See Figure A). | |||
| Note: The LIVMARLI bottle may not be in a box. | |||
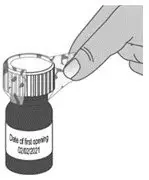
| 2. Write the date (bottle open date) on the LIVMARLI bottle (See Figure B). | |||
| Note: The date of first opening or a throw away (discard) date may already be written on the bottle label by your pharmacist. | |||
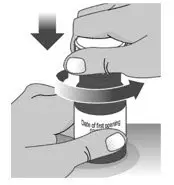
| 3. Remove the plastic seal from the bottle (See Figure C). | |||
| Note: Your pharmacist may have already removed the plastic seal from the bottle. | |||
| Section B: Prepare and give LIVMARLI | |||
| Step 1: Draw the dose | |||
| 1.1: | Open the bottle by pushing down firmly on the child-resistant cap and turning the cap to the left (counter-clockwise) (See Figure D). Do not throw away the child-resistant cap. |
||
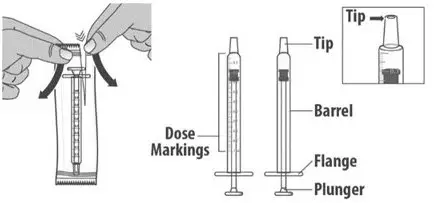
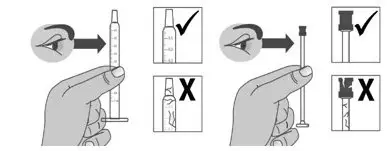
| 1.2: | If using a new dosing dispenser, remove the dosing dispenser from the wrapper (See Figure E). Throw away (dispose of) the wrapper in household trash. If using a used dosing dispenser, make sure the dosing dispenser has been cleaned (See Section C). If there is a cap on the dosing dispenser, remove it and throw away (dispose of) the cap into the household trash (See Figure F). Important:
|
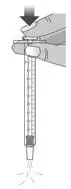
||
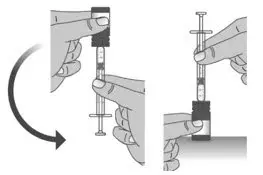
| 1.3: Push the plunger down fully to remove air from the dosing dispenser (See Figure H). | |||
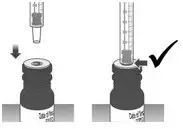
| 1.4: | Make sure the cap is removed from bottle and insert the tip of the dosing dispenser into the bottle. The tip of the dosing dispenser should fit securely into the hole of the bottle (See Figure I). | ||
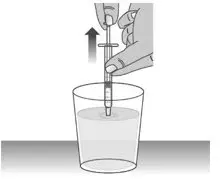
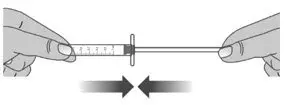
| 1.5: | Keep the dosing dispenser in place and turn the bottle upside down (See Figure J). | ||
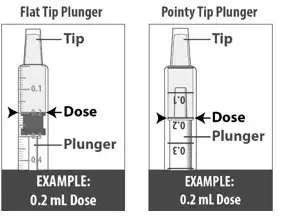
| 1.6: | Pull back the plunger slowly until the top of the plunger is even with the marking on the barrel of the dosing dispenser for your prescribed dose of LIVMARLI (See Figure K). See Figure L on how to align the plunger with your prescribed dose. Note: The medicine should appear colorless to light yellow and clear. If it is not, do not use the medicine and contact your pharmacist. |
||
| Note: Your dose may be different than the dose shown in the figures. | |||
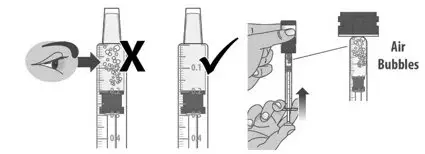
| 1.7: | Check the dosing dispenser for air bubbles. If you see any air bubbles, fully push the plunger so that the medicine flows back into the bottle and withdraw the prescribed dose (See Figure M). | ||
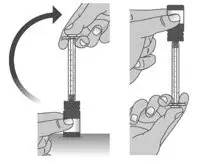
| 1.8: | When you have measured the correct dose, leave the dosing dispenser in the bottle, and turn the bottle right side up (See Figure N). | ||
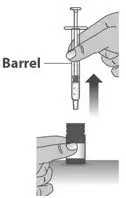
| 1.9: | Carefully remove the dosing dispenser from the bottle by pulling straight up on the barrel of the dosing dispenser (See Figure O). Do not push the dosing dispenser plunger during this step. |
||
| Step 2: Give the dose | |||
| Note: LIVMARLI should be taken while sitting up or standing. After taking LIVMARLI wait a few minutes before lying down. | |||
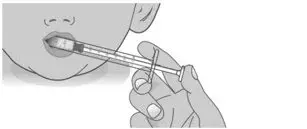
| 2.1: | Place the tip of the dosing dispenser against the inside of the cheek (See Figure P) and slowly push the plunger all the way in to give the entire dose of LIVMARLI (See Figure Q). | ||
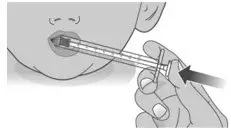
| 2.2: | Swallow the dose. If you are not sure the entire dose was swallowed, do not give another dose. Wait until the next scheduled dose. |
||
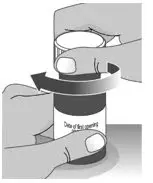
| 2.3: | Place the child-resistant cap back on the bottle and turn the cap to the right (clockwise) (See Figure R). | ||
| Step 1: Rinse dosing dispenser | |||
| 1.1: | Fill a cup with water (See Figure S). | ||
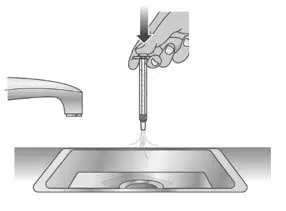
| 1.2: | Clean the dosing dispenser by pulling back on the plunger slowly to fill the dosing dispenser with water from the cup (See Figure T). | ||
| 1.3: | Over a sink, push the water out of the dosing dispenser (See Figure U). Repeat several times to make sure that all of the LIVMARLI has been removed. | ||
| Step 2: Dry the dosing dispenser | |||
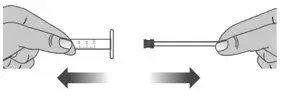
| 2.1: | Remove the plunger from the barrel of the dosing dispenser by pulling the plunger and barrel away from each other (See Figure V). | ||
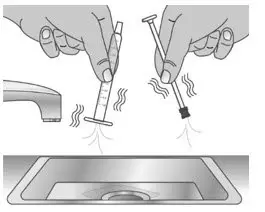
| 2.2: | Shake off excess water (See Figure W). | ||
| 2.3: | Place the plunger and barrel on a clean, dry paper towel to air dry. Store the dosing dispenser in a clean, dry place until your next dose (See Figure X). | ||
|
Before you give the next dose, put the dosing dispenser back together by pushing the plunger into the barrel (See Figure Y). |
|||
|
|||
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Revised:03/2023 | ||
| LB00004v5 | |||
| LIVMARLI
maralixibat chloride solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mirum Pharmaceuticals Inc. (116902386) |