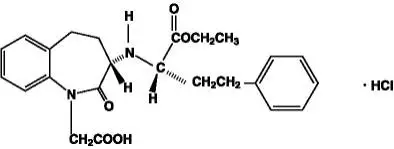
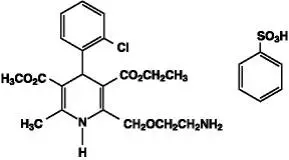
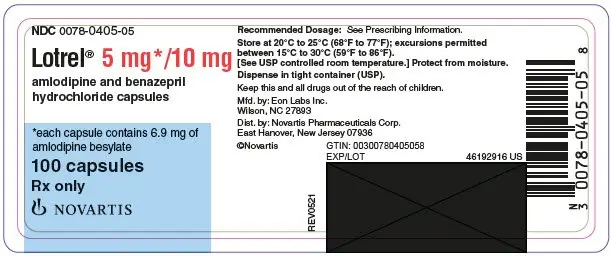
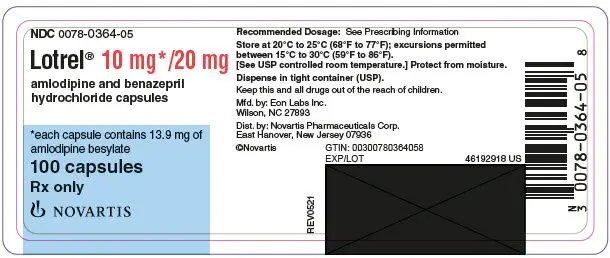
Drug Detail:Lotrel (Amlodipine and benazepril [ am-loe-di-peen-and-ben-ay-ze-pril ])
Drug Class: ACE inhibitors with calcium channel blocking agents
Highlights of Prescribing Information
LOTREL (amlodipine and benazepril hydrochloride) capsules, for oral use
Initial U.S. Approval: 1995
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
When pregnancy is detected, discontinue Lotrel as soon as possible (5.1).
Drugs that act directly on the renin-angiotensin system (RAS) can cause injury and death to the developing fetus (5.1).
Indications and Usage for Lotrel
Lotrel is a combination capsule of amlodipine, a dihydropyridine calcium channel blocker (DHP CCB) and benazepril, an angiotensin-converting enzyme (ACE) inhibitor. Lotrel is indicated for the treatment of hypertension in patients not adequately controlled on monotherapy with either agent. (1)
Lotrel Dosage and Administration
- Usual starting dose is 2.5/10 mg. (2.1)
- May be used as add-on therapy for patients not adequately controlled with either a dihydropyridine calcium channel blocker or an ACE inhibitor (2.2)
- Patients who experience edema with amlodipine may be switched to Lotrel containing a lower dose of amlodipine. (2.1)
Dosage Forms and Strengths
Capsules (amlodipine/benazepril hydrochloride mg): 5/10, 5/20, 10/20, 10/40 (3)
Contraindications
- Do not coadminister aliskiren with ACE inhibitors, including Lotrel, in patients with diabetes. (4)
- Lotrel is contraindicated in patients with a history of angioedema or patients who are hypersensitive to benazepril or to amlodipine. (4)
- Lotrel is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer Lotrel within 36 hours of switching to or from a neprilysin inhibitor, e.g., sacubitril/valsartan. (4)
Warnings and Precautions
- Anaphylactoid reactions, including angioedema (5.2)
- Myocardial infarction or increased angina in patients with obstructive coronary artery disease. (5.3)
- Assess for hypotension and hyperkalemia. (5.4, 5.6)
- Titrate slowly in patients with impaired hepatic or severely impaired renal function. (5.5, 5.7)
Adverse Reactions/Side Effects
Discontinuation because of adverse reactions occurred in 4% of Lotrel-treated patients and 3% of placebo-treated patients. The most common reasons for discontinuation of therapy with Lotrel were cough and edema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Potassium supplements/potassium-sparing diuretics: hyperkalemia (7.1)
- Lithium: Increased serum lithium levels; toxicity symptoms (7.1)
- Injectable gold: facial flushing, nausea, vomiting, hypotension (7.1)
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Risk of renal dysfunction, loss of antihypertensive effect (7.1)
- Do not exceed doses greater than 20 mg daily of simvastatin (7.1)
- mTOR inhibitors: increased risk of angioedema (7.1)
- Dual inhibition of the RAS: Increased risk of renal impairment, hypotension, and hyperkalemia (7.1)
- Neprilysin inhibitors: increased risk of angioedema (7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2021
Related/similar drugs
amlodipine, lisinopril, metoprolol, losartan, furosemide, hydrochlorothiazideFull Prescribing Information
2. Lotrel Dosage and Administration
2.1 General Considerations
The recommended initial dose is amlodipine 2.5 mg/benazepril 10 mg orally once-daily.
Begin therapy with Lotrel only after a patient has either (a) failed to achieve the desired antihypertensive effect with amlodipine or benazepril monotherapy, or (b) demonstrated inability to achieve adequate antihypertensive effect with amlodipine therapy without developing edema.
The antihypertensive effect of Lotrel is largely attained within 2 weeks. If blood pressure remains uncontrolled, the dose may be titrated up to amlodipine 10 mg/benazepril 40 mg once-daily. The dosing should be individualized and adjusted according to the patient’s clinical response.
In clinical trials of amlodipine/benazepril combination therapy using amlodipine doses of 2.5 to 10 mg and benazepril doses of 10 to 40 mg, the antihypertensive effects increased with increasing dose of amlodipine in all patient groups, and the effects increased with increasing dose of benazepril in nonblack groups.
3. Dosage Forms and Strengths
Lotrel (amlodipine/benazepril hydrochloride) capsules are available as follows:
5/10 mg, 5/20 mg, 10/20 mg, and 10/40 mg.
4. Contraindications
- Do not coadminister aliskiren with angiotensin receptor blockers (ARBs), angiotensin-converting enzyme (ACE) inhibitors, including Lotrel in patients with diabetes.
- Lotrel is contraindicated in patients with a history of angioedema, with or without previous ACE inhibitor treatment, or patients who are hypersensitive to benazepril, to any other ACE inhibitor, to amlodipine, or to any of the excipients of Lotrel.
- Lotrel is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer Lotrel within 36 hours of switching to or from a neprilysin inhibitor, e.g., sacubitril/valsartan [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Fetal Toxicity
Lotrel can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Lotrel as soon as possible [see Use in Specific Populations (8.1)].
5.2 Angioedema and Anaphylactoid Reactions
Head and Neck Angioedema: Angioedema of the face, extremities, lips, tongue, glottis, and larynx has been reported in patients treated with benazepril. This may occur at any time during treatment. Angioedema associated with edema of the larynx, tongue, or glottis can compromise the airway and be fatal. If laryngeal stridor or angioedema of the face, tongue, or glottis occurs, discontinue treatment with Lotrel and treat immediately. When involvement of the tongue, glottis, or larynx appears likely to cause airway obstruction, appropriate therapy, e.g., administer subcutaneous epinephrine injection 1:1000 (0.3 to 0.5 mL), promptly [see Adverse Reactions (6)].
Patients with a history of angioedema may be at increased risk for angioedema while receiving Lotrel. Black patients receiving ACE inhibitors have a higher incidence of angioedema compared to nonblacks.
Patients receiving coadministration of ACE inhibitor and mTOR (mammalian target of rapamycin) inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema [see Drug Interactions (7)].
Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Anaphylactoid Reactions During Desensitization: Two patients undergoing desensitizing treatment with hymenoptera (wasp sting) venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions.
Anaphylactoid Reactions During Membrane Exposure: Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
5.3 Increased Angina and/or Myocardial Infarction
Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease.
5.4 Hypotension
Lotrel can cause symptomatic hypotension, sometimes complicated by oliguria, progressive azotemia, acute renal failure, or death. Symptomatic hypotension is most likely to occur in patients who have heart failure, severe aortic or mitral stenosis, obstructive hypertrophic cardiomyopathy or have been volume or salt depleted as a result of diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Correct volume and salt depletion before starting therapy with benazepril. If hypotension occurs, place the patient in the supine position and give physiological saline intravenously if needed. Continue treatment with benazepril once blood pressure and volume have returned to normal.
In patients with congestive heart failure, start Lotrel therapy under close medical supervision; follow closely for the first 2 weeks of treatment and whenever the dose of the benazepril component is increased or a diuretic is added or its dose increased.
In patients undergoing surgery or during anesthesia with agents that produce hypotension, benazepril will block the angiotensin II formation that could otherwise occur secondary to compensatory renin release. Hypotension that occurs as a result of this mechanism can be corrected by volume expansion.
5.5 Impaired Renal Function
Monitor renal function periodically in patients treated with Lotrel. Changes in renal function, including acute renal failure, can be caused by drugs that affect the RAS. Patients whose renal function may depend in part on the activity of the RAS (e.g., patients with renal artery stenosis, severe heart failure, post-myocardial infarction or volume depletion) or who are on Nonsteroidal Anti-Inflammatory Drugs (NSAIDS) or ARBs may be at particular risk of developing acute renal failure on Lotrel. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on Lotrel.
5.6 Hyperkalemia
Monitor serum potassium periodically in patients receiving Lotrel. Drugs that affect the RAS can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements, and/or potassium-containing salt substitutes. In U.S. placebo-controlled trials of Lotrel, hyperkalemia [serum potassium at least 0.5 mEq/L greater than the upper limit of normal (ULN)] not present at baseline occurred in approximately 1.5% of hypertensive patients receiving Lotrel. Increases in serum potassium were generally reversible.
5.7 Hepatitis and Hepatic Failure
There have been rare reports of predominantly cholestatic hepatitis and isolated cases of acute liver failure, some of them fatal, in patients on ACE inhibitors. The mechanism is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevation of hepatic enzymes should discontinue the ACE inhibitor and be kept under medical surveillance.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Lotrel has been evaluated for safety in over 2,991 patients with hypertension; over 500 of these patients were treated for at least 6 months, and over 400 were treated for more than 1 year.
In a pooled analysis of 5 placebo-controlled trials involving Lotrel doses up to 5/20, the reported side effects were generally mild and transient, and there was no relationship between side effects and age, sex, race, or duration of therapy. Discontinuation of therapy due to side effects was required in approximately 4% of patients treated with Lotrel and in 3% of patients treated with placebo.
The most common reasons for discontinuation of therapy with Lotrel in these studies were cough and edema (including angioedema).
The peripheral edema associated with amlodipine use is dose-dependent. When benazepril is added to a regimen of amlodipine, the incidence of edema is substantially reduced.
The addition of benazepril to a regimen of amlodipine should not be expected to provide additional antihypertensive effect in African-Americans. However, all patient groups benefit from the reduction in amlodipine-induced edema.
The side effects considered possibly or probably related to study drug that occurred in these trials in more than 1% of patients treated with Lotrel are shown in the table below. Cough was the only adverse event with at least possible relationship to treatment that was more common on Lotrel (3.3%) than on placebo (0.2%).
| *Edema refers to all edema, such as dependent edema, angioedema, facial edema. | ||||
| Benazepril/Amlodipine | Benazepril | Amlodipine | Placebo | |
| N = 760 | N = 554 | N = 475 | N = 408 | |
| Cough | 3.3 | 1.8 | 0.4 | 0.2 |
| Headache | 2.2 | 3.8 | 2.9 | 5.6 |
| Dizziness | 1.3 | 1.6 | 2.3 | 1.5 |
| Edema* | 2.1 | 0.9 | 5.1 | 2.2 |
The incidence of edema was greater in patients treated with amlodipine monotherapy (5.1%) than in patients treated with Lotrel (2.1%) or placebo (2.2%).
Other side effects considered possibly or probably related to study drug that occurred in U.S. placebo-controlled trials of patients treated with Lotrel or in postmarketing experience were the following:
Body as a Whole: Asthenia and fatigue.
CNS: Insomnia, nervousness, anxiety, tremor, and decreased libido.
Dermatologic: Flushing, hot flashes, rash, skin nodule, and dermatitis.
Digestive: Dry mouth, nausea, abdominal pain, dyspepsia, and esophagitis.
Hematologic: Neutropenia
Musculoskeletal: cramps, and muscle cramps.
Urogenital: Sexual problems, such as impotence, and polyuria.
Monotherapies of benazepril and amlodipine have been evaluated for safety in clinical trials in over 6,000 and 11,000 patients, respectively. The observed adverse reactions to the monotherapies in these trials were similar to those seen in trials of Lotrel.
7. Drug Interactions
7.1 Drug/Drug Interactions
Amlodipine
Simvastatin: Coadministration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients on amlodipine to 20 mg daily.
CYP3A4 Inhibitors: Coadministration with CYP3A inhibitors (moderate and strong) results in increased systemic exposure to amlodipine and may require dose reduction. Monitor for symptoms of hypotension and edema when amlodipine is coadministered with CYP3A4 inhibitors to determine the need for dose adjustment.
CYP3A4 Inducers: No information is available on the quantitative effects of CYP3A4 inducers on amlodipine. Blood pressure should be monitored when amlodipine is coadministered with CYP3A4 inducers (e.g. rifampicin, St. John’s Wort).
Benazepril
Potassium Supplements and Potassium-Sparing Diuretics: Benazepril can attenuate potassium loss caused by thiazide diuretics. Potassium-sparing diuretics (spironolactone, amiloride, triamterene, and others) or potassium supplements can increase the risk of hyperkalemia. If concomitant use of such agents is indicated, the patient’s serum potassium should be monitored frequently.
Lithium: Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving ACE inhibitors during therapy with lithium. When coadministering Lotrel and lithium, frequent monitoring of serum lithium levels is recommended.
Gold: Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors): In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including benazepril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving benazepril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including benazepril, may be attenuated by NSAIDs.
Antidiabetic Agents: In rare cases, diabetic patients receiving an ACE inhibitor (including benazepril) concomitantly with insulin or oral antidiabetics may develop hypoglycemia. Such patients should therefore be advised about the possibility of hypoglycemic reactions and should be monitored accordingly.
Mammalian Target of Rapamycin (mTOR) Inhibitors: The risk of angioedema may be increased in patients receiving coadministration of ACE inhibitors and mTOR inhibitors (e.g., temsirolimus, sirolimus, everolimus).
Dual Blockade of the Renin-Angiotensin System (RAS): Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on Lotrel and other agents that block the RAS.
Do not coadminister aliskiren with Lotrel in patients with diabetes. Avoid use of aliskiren with Lotrel in patients with renal impairment [glomerular filtration rate (GFR) < 60 mL/min].
Neprilysin Inhibitor: Patients taking concomitant neprilysin inhibitors may be at increased risk for angioedema [see Warnings and Precautions (5.1)].
8. Use In Specific Populations
8.2 Lactation
Risk Summary
Minimal amounts of unchanged benazepril and of benazeprilat are excreted into the breast milk of lactating women treated with benazepril, so that a newborn child ingesting nothing but breast milk would receive less than 0.1% of the maternal doses of benazepril and benazeprilat. Limited available data from a published clinical lactation study reports that amlodipine is present in human milk at an estimated median relative infant dose of 4.2%. No adverse effects of amlodipine on the breastfed infant have been observed. There is no available information on the effects of amlodipine or benazepril on milk production.
8.6 Hepatic Impairment
Exposure to amlodipine is increased in patients with hepatic insufficiency, thus consider using lower doses of Lotrel [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
In patients with severe renal impairment systemic exposure to benazepril is increased. The recommended dose of benazepril in this subgroup is 5 mg which is not an available strength with Lotrel. Lotrel is not recommended in patients with severe renal impairment. No dose adjustment of Lotrel is needed in patients with mild or moderate impairment of renal function [see Dosing and Administration (2.2), Warnings and Precautions (5.7) and Clinical Pharmacology (12.3)].
| LOTREL
amlodipine besylate and benazepril hydrochloride capsule |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| LOTREL
amlodipine besylate and benazepril hydrochloride capsule |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| LOTREL
amlodipine besylate and benazepril hydrochloride capsule |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| LOTREL
amlodipine besylate and benazepril hydrochloride capsule |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |