Drug Detail:Lumason (Sulfur hexafluoride [ sul-fur-hex-a-flor-ide ])
Drug Class: Diagnostic radiopharmaceuticals
Highlights of Prescribing Information
LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use
Initial U.S. Approval: 2014
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
See full prescribing information for complete boxed warning
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres (5.1). Most serious reactions occur within 30 minutes of administration (5.1).
- Assess all patients for the presence of any condition that precludes administration (4).
- Always have resuscitation equipment and trained personnel readily available (5.1).
Recent Major Changes
Indications and Usage for Lumason
Lumason is an ultrasound contrast agent indicated for use
- in echocardiography to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border in adult and pediatric patients with suboptimal echocardiograms (1)
- in ultrasonography of the liver for characterization of focal liver lesions in adult and pediatric patients (1)
- in ultrasonography of the urinary tract for the evaluation of suspected or known vesicoureteral reflux in pediatric patients (1)
Lumason Dosage and Administration
Avoid intra-arterial injection (2.1, 5.3)
See Full Prescribing Information for reconstitution instructions (2.3)
For intravenous injection:
- Echocardiography in adults: After reconstitution, administer 2 mL as an intravenous injection (2.2, 2.4)
- Echocardiography in pediatric patients: After reconstitution, administer 0.03 mL per kg as an intravenous injection up to a maximum of 2 mL per injection (2.2, 2.4)
- Ultrasonography of the liver in adults: After reconstitution, administer 2.4 mL as an intravenous injection (2.2, 2.4)
- Ultrasonography of the liver in pediatric patients: After reconstitution, administer 0.03 mL per kg as an intravenous injection, up to a maximum of 2.4 mL per injection (2.2, 2.4)
- May repeat dose one time during a single examination (2.2, 2.4)
- Follow each injection with an intravenous flush of 0.9% Sodium Chloride Injection, USP (2.2, 2.4)
For intravesical administration in pediatric patients:
- Ultrasonography of the urinary tract: After reconstitution, administer 1 mL via sterile 6 to 8F urinary catheter. Bladder should be first emptied and then partially filled with 0.9% Sodium Chloride Injection, USP before injection of Lumason (2.2, 2.4)
- After Lumason administration, continue filling the bladder with 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion (2.4)
Dosage Forms and Strengths
- For injectable suspension: 25 mg of lipid-type A lyophilized powder with headspace fill of 60.7 mg sulfur hexafluoride in a single-patient use vial for reconstitution (3)
Contraindications
- Hypersensitivity to sulfur hexafluoride lipid microspheres or its components, such as polyethylene glycol (PEG) (4)
Warnings and Precautions
- Cardiopulmonary reactions, including fatalities. Always have resuscitation equipment and trained personnel readily available (5.1)
- Hypersensitivity reactions. Serious acute hypersensitivity reactions have occurred in patients with no prior exposure to sulfur hexafluoride lipid-containing microsphere products, including patients with prior hypersensitivity reaction(s) to PEG (5.2, 6)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 0.5%) are headache and nausea (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Bracco Diagnostics Inc at 1-800-257-5181 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2023
Full Prescribing Information
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
- Assess all patients for the presence of any condition that precludes administration [see Contraindications (4)].
- Always have resuscitation equipment and trained personnel readily available [see Warnings and Precautions (5.1)].
Lumason Dosage and Administration
2.1 Important Administration Instructions
Do not administer Lumason by intra-arterial injection [see Warnings and Precautions (5.3)].
2.3 Reconstitution Instructions
- Refer to Section 2.3.1 for instructions for using the single patient use kit with diluent provided
- Refer to Section 2.3.2 for instructions for using the 20-vial pack without diluent provided
2.3.1 Lumason Kit (single patient use kit)
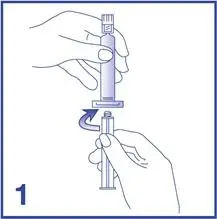
- Inspect the Lumason kit and its components for signs of damage. Do not use the kit if the protective caps on the Lumason vial and prefilled syringe with 5 mL 0.9% Sodium Chloride Injection, USP are not intact or if the kit shows other signs of damage.
- Under aseptic conditions, reconstitute the Lumason vial using the following illustrated steps:
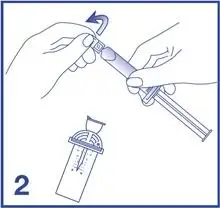
2. Open the Mini-Spike blister and remove the syringe tip cap (see Figure 2).
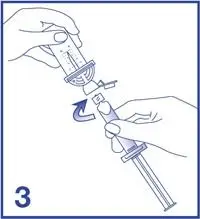

5. Empty the content of the syringe into the vial by pushing on the plunger rod (see Figure 5).
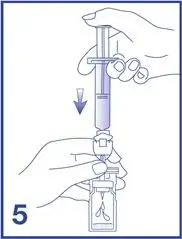
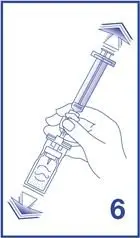
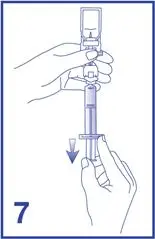
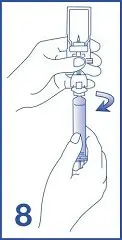
- Following reconstitution, Lumason suspension contains 1.5 to 5.6 x108 microspheres/mL with 45 mcg/mL of sulfur hexafluoride.
- Use immediately after reconstitution. If the suspension is not used immediately after reconstitution, resuspend the microspheres for a few seconds by hand agitation before the suspension is drawn into the syringe. Reconstituted suspension within a vial may be used for up to 3 hours from the time of its reconstitution. Maintain the vial containing the reconstituted suspension at room temperature 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
2.3.2 Lumason Pack (20-vial pack)

Lumason vials are to be used with the supplied Mini-Spike only.
Use only additive-free 0.9% Sodium Chloride Injection, USP for the reconstitution of Lumason.
- Inspect the Lumason components for signs of damage. Do not use the Lumason vial if the protective cap on the vial is not intact or other components in the pack show signs of damage.
- Use aseptic conditions for the preparation and administration of Lumason.
- Two healthcare professionals (HCPs) should verify that the
solution selected for reconstitution of Lumason is additive-free 0.9%
Sodium Chloride Injection, USP.
- Ensure that any air in the syringe is expelled.
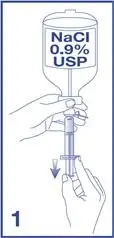
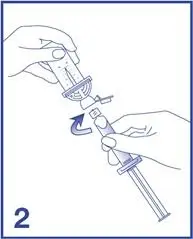

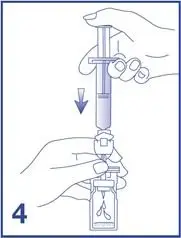
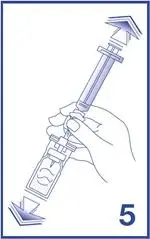
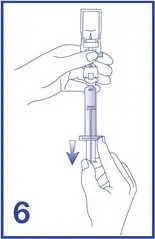
7. Unscrew the syringe from the Mini-Spike (see Figure 7).
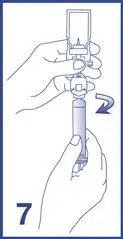
8. Label the syringe using the peel-off sticker provided.
- Following reconstitution, Lumason suspension contains 1.5 to 5.6 x108 microspheres/mL with 45 mcg/mL of sulfur hexafluoride.
- Use immediately after reconstitution. If the suspension is not used immediately after reconstitution, resuspend the microspheres for a few seconds by hand agitation before the suspension is drawn into the syringe. Reconstituted suspension within a vial may be used for up to 3 hours from the time of its reconstitution. Maintain the vial containing the reconstituted suspension at room temperature 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
2.4 Administration Instructions
Administer Lumason as an intravenous bolus injection.
Intravesical Administration in Pediatric Patients
- Insert a sterile 6 French to 8 French urinary catheter into the bladder under sterile conditions;
- Empty the bladder of urine, and then fill the bladder with sterile 0.9% Sodium Chloride Injection, USP to approximately one third or half of its predicted total volume. The total bladder volume in children is calculated as [(age in years + 2) x 30] mL;
- Administer Lumason as an intravesical bolus injection through the urinary catheter;
- Continue filling the bladder wIith 0.9% Sodium Chloride Injection, USP until the patient has the urge to micturate or at the first sign of back pressure to the infusion.
- Immediately following the first voiding, the bladder may be refilled with 0.9% Sodium Chloride Injection, USP for a second cycle of voiding and imaging, without the need of a second Lumason administration.
Dosage Forms and Strengths
3-part single patient use kit comprised of:
- one Lumason clear vial containing 25 mg of lipid-type A sterile white lyophilized powder with headspace filled with 60.7 mg of sulfur hexafluoride gas
- one prefilled syringe containing 5 mL of 0.9% Sodium Chloride Injection, USP (Diluent)
- one Mini-Spike
- twenty Lumason clear vials, each containing 25 mg of lipid-type A sterile white lyophilized powder with headspace filled with 60.7 mg of sulfur hexafluoride gas
- twenty Mini-Spikes
- twenty peel-off syringe labels
Contraindications
Lumason is contraindicated in patients with known or suspected:
- Hypersensitivity to sulfur hexafluoride lipid microsphere or its components, such as polyethylene glycol (PEG) [see Warnings and Precautions (5.2) and Description (11)].
Warnings and Precautions
Adverse Reactions/Side Effects
The following serious adverse reactions are discussed elsewhere in the labeling:
- Cardiopulmonary reactions [see Warnings and Precautions (5.1)]
- Hypersensitivity reactions [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
| *occurring in at least 0.2% of patients | |
| Table 1. Adverse Reactions in Adult Patients* n = 6856 |
|
| Number (%) of Patients with Adverse Reactions | 340 (5%) |
| Headache | 65 (1%) |
| Nausea | 37 (0.5%) |
| Dysgeusia | 29 (0.4%) |
| Injection site pain | 23 (0.3%) |
| Feeling Hot | 18 (0.3%) |
| Chest discomfort | 17 (0.2%) |
| Chest pain | 12 (0.2%) |
| Dizziness | 11 (0.2%) |
| Injection Site Warmth | 11 (0.2%) |
Use In Specific Populations
Lumason Description
- The single patient use kit contains the following three
items:
- one clear glass 10 mL vial containing 25 mg of white lyophilized powder lipid-type A, 60.7 mg of sulfur hexafluoride gas and capped with a blue flip-cap
- one prefilled syringe containing 5 mL 0.9% Sodium Chloride
Injection, USP (Diluent)
Each prefilled syringe with 5 mL of diluent 0.9% Sodium Chloride Injection, USP is sterile, nonpyrogenic, and additive-free containing 9 mg sodium chloride per mL. - one Mini-Spike
- The 20-vial pack is comprised of:
- twenty Lumason clear vials, each containing 25 mg of lipid-type A sterile white lyophilized powder with headspace filled with 60.7 mg of sulfur hexafluoride gas
- twenty Mini-Spikes
- twenty peel-off syringe labels
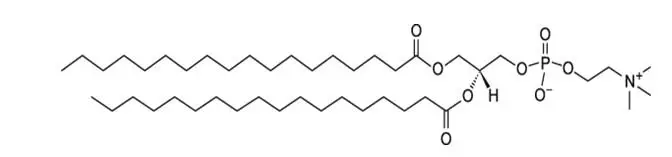
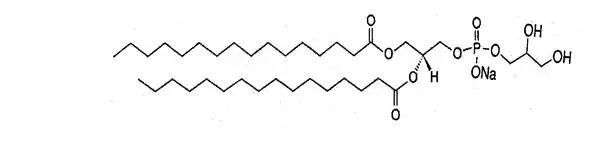
Sulfur hexafluoride has a molecular weight of 145.9 and the following chemical structure:

The sulfur hexafluoride lipid microsphere characteristics are listed in Table 2:
| Table 2. Microsphere Characteristics | |
| Mean diameter range | 1.5 – 2.5 μm |
| Percent of microspheres ≤ 10 µm | ≥ 99% |
| Upper size limit | 100.0% ≤ 20 µm |
Lumason - Clinical Pharmacology
Clinical Studies
14.1 Echocardiography
Endocardial Border Delineation and Duration of Useful Contrast Effect
| Table 3. Reduction in Percentage of Patients with Inadequate Border Delineation | ||||||
| Reader | Study A N = 76 | Study B N = 62 | Study C N = 53 |
|||
| Non- contrast | Lumason | Non- contrast | Lumason | Non- contrast | Lumason | |
| A | 60 (79%) | 22 (33%) | 31 (50%) | 12 (19%) | 12 (23%) | 10 (19%) |
| B | 62 (82%) | 29 (37%) | 54 (87%) | 6 (10%) | 45 (85%) | 20 (38%) |
Left Ventricular Opacification
| a Reader A had missing segment data with contrast echocardiography for one patient; b Reader B had missing segment data with non-contrast echocardiography for one patient; bb Reader B had missing segment data with contrast echocardiography for three patients; c Reader C had missing segment data with contrast echocardiography for one patient | |||
| Table 4. Number of Pediatric Patients with Inadequate Border Delineation with and without Lumason | |||
| Reader A | Reader B | Reader C | |
| Non-contrast | 12/12 | 11/11b | 12/12 |
| Lumason | 1/11a | 0/9bb | 0/11c |
14.2 Ultrasonography of the Liver
Truth standard included: histology/surgery, contrast CT, contrast MRI, and/or 6 month follow-up.
| * Statistically significant improvement from non-contrast (p<0.05 based on McNemar’s test) | ||||||
| Table 5. Diagnostic Performance of Lumason Ultrasound for Characterization of Focal Liver Lesions | ||||||
| Study A: | ||||||
| Sensitivity
(patients with malignant lesions) N=119 | Specificity
(patients with benign lesions) N=140 |
|||||
| Lumason % | Non-contrast % | Difference (95% CI) | Lumason % | Non-contrast % | Difference (95% CI) | |
| Reader 1 | 87* | 49 | 38 (30, 54) | 71 | 63 | 8 (-4, 21) |
| Reader 2 | 76* | 35 | 41 (29, 52) | 83* | 54 | 29 (21, 44) |
| Reader 3 | 92* | 16 | 76 (67, 84) | 73* | 22 | 51 (40, 61) |
| Study B: | ||||||
| Sensitivity
(patients with malignant lesions) N=124 | Specificity
(patients with benign lesions) N=116 |
|||||
| Lumason % | Non-contrast % | Difference (95% CI) | Lumason % | Non-contrast % | Difference (95% CI) | |
| Reader 4 | 65 | 53 | 12 (-1, 23) | 72* | 24 | 48 (35, 58) |
| Reader 5 | 61* | 41 | 20 (7, 32) | 67* | 7 | 60 (50, 70) |
| Reader 6 | 47 | 66 | -19 (-31, -7) | 88* | 59 | 29 (18, 40) |
How is Lumason supplied
16.1 How Supplied
-
5 single patient use kits (NDC 0270-7099-16) with each kit containing:
- One Lumason vial of 25 mg lipid-type A white lyophilized powder with headspace fill of 60.7 mg of sulfur hexafluoride
- One prefilled syringe containing 5mL of 0.9% Sodium Chloride Injection, USP (Diluent)
- One Mini-Spike
-
20-vial pack (NDC 0270-7097-07) containing:
- Twenty (20) Lumason vials of 25 mg lipid-type A white lyophilized powder with headspace fill of 60.7 mg of sulfur hexafluoride
- Twenty (20) Mini-Spikes
- Twenty (20) peel-off syringe labels
| LUMASON
sulfur hexafluoride kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LUMASON
sulfur hexafluoride injection, powder, lyophilized, for suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - BRACCO DIAGNOSTICS INC (849234661) |
| Registrant - BRACCO DIAGNOSTICS INC (849234661) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bracco Suisse SA | 485635705 | MANUFACTURE(0270-7097, 0270-7099) , ANALYSIS(0270-7099, 0270-7097) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma-Fertigung GmbH & Co. KG | 316126754 | MANUFACTURE(0270-7097, 0270-7099) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bracco Imaging SPA | 434384008 | MANUFACTURE(0270-7097, 0270-7099) , ANALYSIS(0270-7099, 0270-7097) | |







.webp)
.webp)
