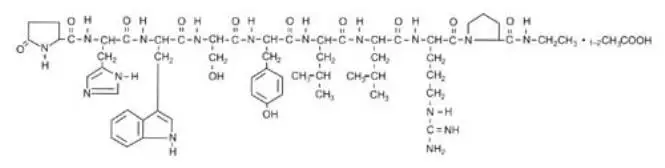
Drug Detail:Lupron depot (Leuprolide [ loo-proe-lide ])
Drug Class: Gonadotropin releasing hormones Hormones / antineoplastics
Highlights of Prescribing Information
LUPRON DEPOT (leuprolide acetate for depot suspension)
Initial U.S. Approval: 1989
Recent Major Changes
| Indication and Usage (1) | 04/2022 |
Indications and Usage for Lupron Depot
LUPRON DEPOT is a gonadotropin releasing hormone (GnRH) agonist indicated for:
- treatment of advanced prostatic cancer. (1)
Lupron Depot Dosage and Administration
LUPRON DEPOT must be administered under the supervision of a physician. Due to different release characteristics, the dosage strengths are not additive and must be selected based upon the desired dosing schedule. (2)
- LUPRON DEPOT 7.5 mg for 1-month administration, given as a single intramuscular injection every 4 weeks. (2.1)
- LUPRON DEPOT 22.5 mg for 3-month administration, given as a single intramuscular injection every 12 weeks. (2.2)
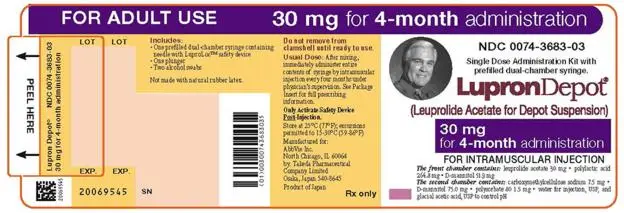
- LUPRON DEPOT 30 mg for 4-month administration, given as a single intramuscular injection every 16 weeks. (2.3)
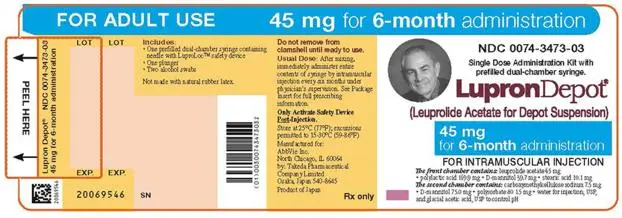
- LUPRON DEPOT 45 mg for 6-month administration, given as a single intramuscular injection every 24 weeks. (2.4)
Dosage Forms and Strengths
7.5 mg, 22.5 mg, 30 mg, and 45 mg injections in a kit with prefilled dual chamber syringe. (3)
Contraindications
- Hypersensitivity to GnRH, GnRH agonist or any of the excipients in LUPRON DEPOT. (4)
Warnings and Precautions
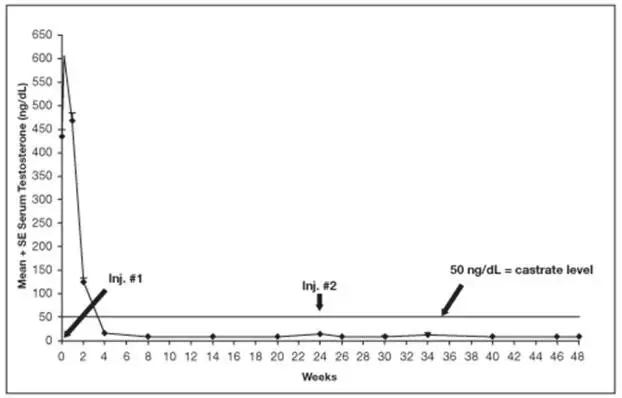
- Increased serum testosterone (~ 50% above baseline) during first week of treatment; monitor serum testosterone and PSA. (5.1, 5.6)
○ Isolated cases of transient worsening of symptoms, or additional signs and symptoms of prostate cancer during the first few weeks of treatment. (5.1)
○ A small number of patients may experience a temporary increase in bone pain which can be managed symptomatically. (5.1)
○ Isolated cases of ureteral obstruction and spinal cord compression have been reported with GnRH agonists, which may contribute to paralysis with or without fatal complications. (5.1)
- Hyperglycemia and Diabetes: Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH analogs. Monitor blood glucose level and manage according to current clinical practice. (5.2)
- Cardiovascular Diseases: Increased risk of myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH analogs in men. Monitor for cardiovascular disease and manage according to current clinical practice. (5.3)
- Effect on QT/QTc Interval: Androgen deprivation therapy may prolong the QT interval. Consider risks and benefits. (5.4)
- Convulsions have been observed in patients with or without a history of predisposing factors. Manage convulsions according to the current clinical practice. (5.5)
- Embryo-Fetal Toxicity: LUPRON DEPOT may cause fetal harm. (5.7, 8.1)
Adverse Reactions/Side Effects
- LUPRON DEPOT 7.5 mg for 1-month administration: The most common adverse reactions (>10%) were general pain, hot flashes/sweats, GI disorders, edema, respiratory disorder, urinary disorder. (6.1)
- LUPRON DEPOT 22.5 mg for 3-month administration: The most common adverse reactions (>10%) were general pain, injection site reaction, hot flashes/sweats, GI disorders, joint disorders, testicular atrophy, urinary disorders. (6.2)
- LUPRON DEPOT 30 mg for 4-month administration: The most common adverse reactions (>10%) were asthenia, flu syndrome, general pain, headache, injection site reaction, hot flashes/sweats, GI disorders, edema, skin reaction, urinary disorders. (6.3)
- LUPRON DEPOT 45 mg for 6-month administration: The most common adverse reactions (>10%) were hot flush, injection site pain, upper respiratory infection, and fatigue. (6.4)
In postmarketing experience, mood swings, depression, rare reports of suicidal ideation and attempt, rare reports of pituitary apoplexy, and rare reports of serious drug-induced liver injury have been reported. (6.5)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Use In Specific Populations
- Females and males of reproductive potential: LUPRON DEPOT may impair fertility. Counsel patients on pregnancy planning and prevention. (8.3)
- Pediatric: These LUPRON DEPOT formulations are not indicated for use in children. See the LUPRON DEPOT PED®package insert for the use of leuprolide acetate in children with central precocious puberty.
- Geriatric: This label reflects clinical trials for LUPRON DEPOT in prostate cancer in which the majority of the subjects studied were at least 65 years of age.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2022
Full Prescribing Information
1. Indications and Usage for Lupron Depot
LUPRON DEPOT 7.5 mg for 1-month administration, 22.5 mg for 3-month administration, 30 mg for 4-month administration, and 45 mg for 6-month administration (leuprolide acetate) are indicated for the treatment of advanced prostatic cancer.
2. Lupron Depot Dosage and Administration
LUPRON DEPOT must be administered under the supervision of a physician.
In patients treated with GnRH analogues for prostate cancer, treatment is usually continued upon development of non-metastatic and metastatic castration-resistant prostate cancer.
| Dosage | 7.5 mg for 1-Month Administration | 22.5 mg for 3-Month Administration | 30 mg for 4-Month Administration | 45 mg for 6-Month Administration |
| Recommended dose | 1 injection every 4 weeks | 1 injection every 12 weeks | 1 injection every 16 weeks | 1 injection every 24 weeks |
2.1 LUPRON DEPOT 7.5 mg for 1-Month Administration
The recommended dose of LUPRON DEPOT 7.5 mg for 1-month administration is one injection every 4 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and should be administered every 4 weeks as a single intramuscular injection.
For optimal performance of the prefilled dual chamber syringe (PDS), read and follow the instructions in Section 2.5.
2.2 LUPRON DEPOT 22.5 mg for 3-Month Administration
The recommended dose of LUPRON DEPOT 22.5 mg for 3-month administration is one injection every 12 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and should be administered every 12 weeks as a single intramuscular injection.
For optimal performance of the prefilled dual chamber syringe (PDS), read and follow the instructions in Section 2.5.
2.3 LUPRON DEPOT 30 mg for 4-Month Administration
The recommended dose of LUPRON DEPOT 30 mg for 4-month administration is one injection every 16 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and should be administered every 16 weeks as a single intramuscular injection.
For optimal performance of the prefilled dual chamber syringe (PDS), read and follow the instructions in Section 2.5.
2.4 LUPRON DEPOT 45 mg for 6-Month Administration
The recommended dose of LUPRON DEPOT 45 mg for 6-month administration is one injection every 24 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and should be administered every 24 weeks as a single intramuscular injection.
For optimal performance of the prefilled dual chamber syringe (PDS), read and follow the instructions in Section 2.5.
2.5 Reconstitution and Administration for Injection of LUPRON DEPOT
- Reconstitute and administer the lyophilized microspheres as a single intramuscular injection.
- Inject the suspension immediately or discard if not used within two hours, because LUPRON DEPOT does not contain a preservative.
1. Visually inspect the LUPRON DEPOT powder. DO NOT USE the syringe if clumping or caking is evident. A thin layer of powder on the wall of the syringe is considered normal prior to mixing with the diluent. The diluent should appear clear and colorless.
2. To prepare for injection, screw the white plunger into the end stopper until the stopper begins to turn (see Figure 1 and Figure 2).
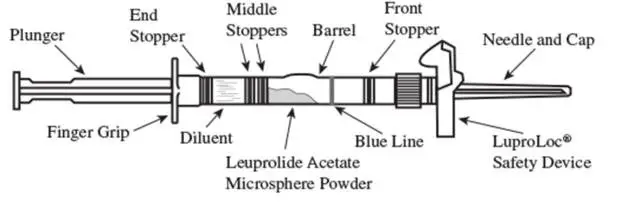
Figure 1
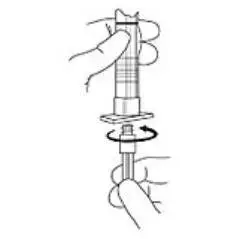
Figure 2
3. Hold the syringe UPRIGHT. Release the diluent by SLOWLY PUSHING (6 to 8 seconds) the plunger until the first middle stopper is at the blue line in the middle of the barrel (see Figure 3).
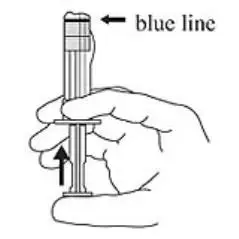
Figure 3
4. Keep the syringe UPRIGHT. Mix the microspheres (powder) thoroughly by gently shaking the syringe until the powder forms a uniform suspension. The suspension will appear milky. If the powder adheres to the stopper or caking/clumping is present, tap the syringe with your finger to disperse. DO NOT USE if any of the powder has not gone into suspension (see Figure 4).
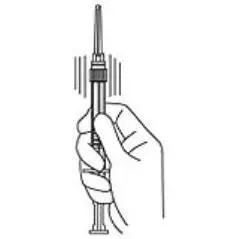
Figure 4
5. Keep the syringe UPRIGHT. With the opposite hand pull the needle cap upward without twisting.
6. Keep the syringe UPRIGHT. Advance the plunger to expel the air from the syringe. Now the syringe is ready for injection.
7. After cleaning the injection site with an alcohol swab, administer the intramuscular injection by inserting the needle at a 90 degree angle into the gluteal area, anterior thigh, or deltoid; injection sites should be alternated (see Figure 5).
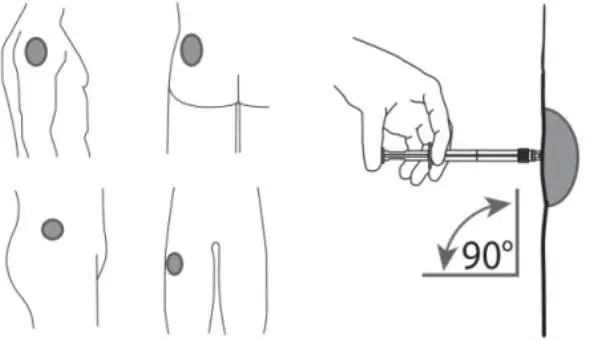
Figure 5
NOTE: If a blood vessel is accidentally penetrated, aspirated blood will be visible just below the luer lock (see Figure 6) and can be seen through the transparent LuproLoc® safety device. If blood is present, remove the needle immediately. Do not inject the medication.
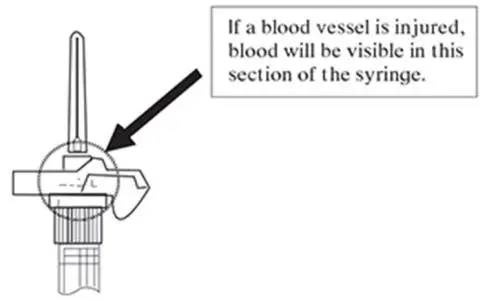
Figure 6
8. Inject the entire contents of the syringe intramuscularly.
9. Withdraw the needle. Once the syringe has been withdrawn, immediately activate the LuproLoc® safety device by pushing the arrow on the lock upward towards the needle tip with the thumb or finger, as illustrated, until the needle cover of the safety device over the needle is fully extended and a CLICK is heard or felt (see Figure 7).
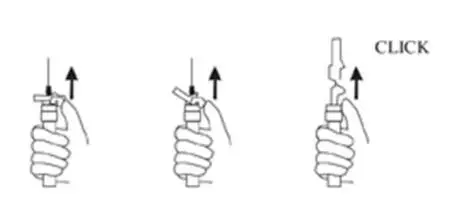
Figure 7
10. Dispose of the syringe according to local regulations/procedures.
3. Dosage Forms and Strengths
LUPRON DEPOT 7.5 mg for 1-month administration, 22.5 mg for 3-month administration, 30 mg for 4-month administration, and 45 mg for 6-month administration are each supplied as a kit with prefilled dual chamber syringe.
4. Contraindications
LUPRON DEPOT is contraindicated in:
- Hypersensitivity
LUPRON DEPOT is contraindicated in individuals with known hypersensitivity to GnRH agonists or any of the excipients in LUPRON DEPOT. Reports of anaphylactic reactions to GnRH agonists have been reported in the medical literature.
5. Warnings and Precautions
5.1 Tumor Flare
Initially, LUPRON DEPOT, like other GnRH agonists, causes increases in serum levels of testosterone to approximately 50% above baseline during the first weeks of treatment. Isolated cases of ureteral obstruction and spinal cord compression have been observed, which may contribute to paralysis with or without fatal complications. Transient worsening of symptoms may develop. A small number of patients may experience a temporary increase in bone pain, which can be managed symptomatically.
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy.
5.2 Hyperglycemia and Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
5.3 Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
5.4 Effect on QT/QTc Interval
Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
5.5 Convulsions
Postmarketing reports of convulsions have been observed in patients on leuprolide acetate therapy. These included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above. Patients receiving a GnRH agonist who experience convulsion should be managed according to current clinical practice.
5.6 Laboratory Tests
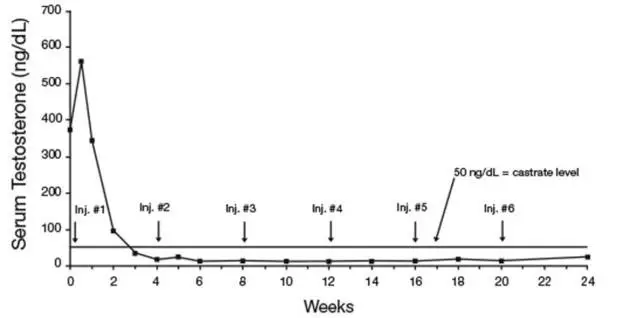
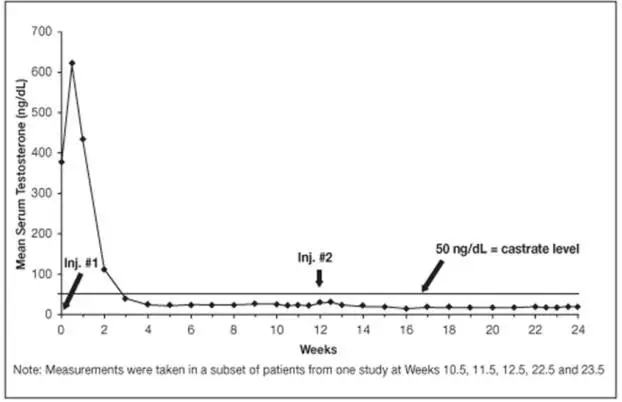
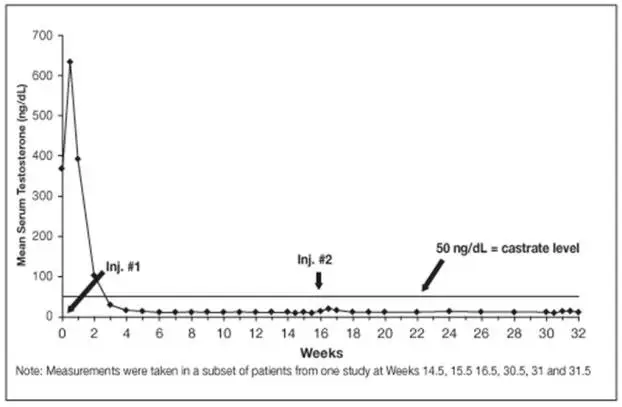
Monitor serum levels of testosterone following injection of LUPRON DEPOT 7.5 mg for 1-month administration, 22.5 mg for 3-month administration, 30 mg for 4-month administration, or 45 mg for 6-month administration. In the majority of patients, testosterone levels increased above baseline, and then declined thereafter to castrate levels (< 50 ng/dL) within four weeks. [see Clinical Studies (14) and Adverse Reactions (6)].
5.7 Embryo-Fetal Toxicity
Based on findings in animal studies, LUPRON DEPOT may cause fetal harm when administered to a pregnant woman. In animal developmental and reproductive toxicology studies, administration of the monthly formulation of leuprolide acetate on day 6 of pregnancy (sustained exposure was expected throughout the period of organogenesis) caused adverse embryo-fetal toxicity in animals at doses less than the human dose, based on body surface area, using an estimated daily dose. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus [see Use in Specific Populations (8.1)].
6. Adverse Reactions/Side Effects
The following is discussed in more detail in other sections of the labeling:
- Tumor Flare [see Warnings and Precautions (5.1)]
- Hyperglycemia and Diabetes [see Warnings and Precautions (5.2)]
- Cardiovascular Disease [see Warnings and Precautions (5.3)]
- Effect on QT/QTc Interval [see Warnings and Precautions (5.4)]
- Convulsions [see Warnings and Precautions (5.5)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 LUPRON DEPOT 7.5 mg for 1-Month Administration
In the majority of patients testosterone levels increased above baseline during the first week, declining thereafter to baseline levels or below by the end of the second week of treatment.
Potential exacerbations of signs and symptoms during the first few weeks of treatment is a concern in patients with vertebral metastases and/or urinary obstruction or hematuria which, if aggravated, may lead to neurological problems such as temporary weakness and/or paresthesia of the lower limbs or worsening of urinary symptoms [see Warnings and Precautions (5.1)].
In a clinical trial of LUPRON DEPOT 7.5 mg for 1-month administration, the following adverse reactions were reported in 5% or more of the patients during the initial 24-week treatment period.
| Table 2. Adverse Reactions Reported in ≥ 5% of Patients | ||
| LUPRON DEPOT 7.5 mg for 1-Month Administration (N=56) | ||
| N | (%) | |
| Body As A Whole | ||
| General pain | 13 | (23.2) |
| Infection | 3 | (5.4) |
| Cardiovascular System | ||
| Hot flashes/sweats* | 32 | (57.1) |
| Digestive System | ||
| GI disorders | 8 | (14.3) |
| Metabolic and Nutritional Disorders | ||
| Edema | 8 | (14.3) |
| Nervous System | ||
| Libido decreased* | 3 | (5.4) |
| Respiratory System | ||
| Respiratory disorder | 6 | (10.7) |
| Urogenital System | ||
| Urinary disorder | 7 | (12.5) |
| Impotence* | 3 | (5.4) |
| Testicular atrophy* | 3 | (5.4) |
| * Due to the expected physiologic effect of decreased testosterone levels. | ||
In this same study, the following adverse reactions were reported in less than 5% of the patients on LUPRON DEPOT 7.5 mg for 1-month administration.
Body As A Whole - Asthenia, Cellulitis, Fever, Headache, Injection site reaction, Neoplasm
Cardiovascular System - Angina, Congestive heart failure
Digestive System - Anorexia, Dysphagia, Eructation, Peptic ulcer
Hemic and Lymphatic System - Ecchymosis
Musculoskeletal System - Myalgia
Nervous System - Agitation, Insomnia/sleep disorders, Neuromuscular disorders
Respiratory System - Emphysema, Hemoptysis, Lung edema, Sputum increased
Skin and Appendages - Hair disorder, Skin reaction
Urogenital System - Balanitis, Breast enlargement, Urinary tract infection
Laboratory Abnormalities
Abnormalities of certain parameters were observed, but their relationship to drug treatment are difficult to assess in this population. The following were recorded in ≥5% of patients at final visit: Decreased albumin, decreased hemoglobin/hematocrit, decreased prostatic acid phosphatase, decreased total protein, decreased urine specific gravity, hyperglycemia, hyperuricemia, increased BUN, increased creatinine, increased liver function tests (AST, LDH), increased phosphorus, increased platelets, increased prostatic acid phosphatase, increased total cholesterol, increased urine specific gravity, leukopenia.
6.2 LUPRON DEPOT 22.5 mg for 3-Month Administration
In two clinical trials of LUPRON DEPOT 22.5 mg for 3-month administration, the following adverse reactions were reported to have a possible or probable relationship to drug as ascribed by the treating physician in 5% or more of the patients receiving the drug. Often, causality is difficult to assess in patients with metastatic prostate cancer. Reactions considered not drug-related are excluded.
| Table 3. Adverse Reactions Reported in ≥ 5% of Patients | ||
| LUPRON DEPOT 22.5 mg for 3-Month Administration | ||
| Body System/Reaction | N=94 | (%) |
| Body As A Whole | ||
| Asthenia | 7 | (7.4) |
| General Pain | 25 | (26.6) |
| Headache | 6 | (6.4) |
| Injection Site Reaction | 13 | (13.8) |
| Cardiovascular System | ||
| Hot flashes/Sweats | 55 | (58.5) |
| Digestive System | ||
| GI Disorders | 15 | (16.0) |
| Musculoskeletal System | ||
| Joint Disorders | 11 | (11.7) |
| Central/Peripheral Nervous System | ||
| Dizziness/Vertigo | 6 | (6.4) |
| Insomnia/Sleep Disorders | 8 | (8.5) |
| Neuromuscular Disorders | 9 | (9.6) |
| Respiratory System | ||
| Respiratory Disorders | 6 | (6.4) |
| Skin and Appendages | ||
| Skin Reaction | 8 | (8.5) |
| Urogenital System | ||
| Testicular Atrophy | 19 | (20.2) |
| Urinary Disorders | 14 | (14.9) |
In these same studies, the following adverse reactions were reported in less than 5% of the patients on LUPRON DEPOT 22.5 mg for 3-month administration.
Body As A Whole - Enlarged abdomen, Fever
Cardiovascular System - Arrhythmia, Bradycardia, Heart failure, Hypertension, Hypotension, Varicose vein
Digestive System - Anorexia, Duodenal ulcer, Increased appetite, Thirst/dry mouth
Hemic and Lymphatic System - Anemia, Lymphedema
Metabolic and Nutritional Disorders - Dehydration, Edema
Central/Peripheral Nervous System - Anxiety, Delusions, Depression, Hypesthesia, Libido decreased*, Nervousness, Paresthesia
Respiratory System - Epistaxis, Pharyngitis, Pleural effusion, Pneumonia
Special Senses - Abnormal vision, Amblyopia, Dry eyes, Tinnitus
Urogenital System - Gynecomastia, Impotence*, Penis disorders, Testis disorders.
* Physiologic effect of decreased testosterone.
Laboratory Abnormalities
Abnormalities of certain parameters were observed, but are difficult to assess in this population. The following were recorded in ≥5% of patients: Increased BUN, Hyperglycemia, Hyperlipidemia (total cholesterol, LDL-cholesterol, triglycerides), Hyperphosphatemia, Abnormal liver function tests, Increased PT, Increased PTT. Additional laboratory abnormalities reported were: Decreased platelets, Decreased potassium and Increased WBC.
6.3 LUPRON DEPOT 30 mg for 4-Month Administration
The 4-month formulation of LUPRON DEPOT 30 mg was utilized in clinical trials that studied the drug in 49 nonorchiectomized prostate cancer patients for 32 weeks or longer and in 24 orchiectomized prostate cancer patients for 20 weeks.
In the above described clinical trials, the following adverse reactions were reported in ≥ 5% of the patients during the treatment period.
| Table 4. Adverse Reactions Reported in ≥ 5% of Patients | ||||
| LUPRON DEPOT 30 mg for 4-Month Administration | ||||
| Body System/Events | Nonorchiectomized | Orchiectomized | ||
| Study 013 | Study 012 | |||
| N=49 | (%) | N=24 | (%) | |
| Body As A Whole | ||||
| Asthenia | 6 | (12.2) | 1 | (4.2) |
| Flu Syndrome | 6 | (12.2) | 0 | (0.0) |
| General Pain | 16 | (32.7) | 1 | (4.2) |
| Headache | 5 | (10.2) | 1 | (4.2) |
| Injection Site Reaction | 4 | (8.2) | 9 | (37.5) |
| Cardiovascular System | ||||
| Hot flashes/Sweats | 23 | (46.9) | 2 | (8.3) |
| Digestive System | ||||
| GI Disorders | 5 | (10.2) | 3 | (12.5) |
| Metabolic and Nutritional Disorders | ||||
| Dehydration | 4 | (8.2) | 0 | (0.0) |
| Edema | 4 | (8.2) | 5 | (20.8) |
| Musculoskeletal System | ||||
| Joint Disorder | 8 | (16.3) | 1 | (4.2) |
| Myalgia | 4 | (8.2) | 0 | (0.0) |
| Nervous System | ||||
| Dizziness/Vertigo | 3 | (6.1) | 2 | (8.3) |
| Neuromuscular Disorders | 3 | (6.1) | 1 | (4.2) |
| Paresthesia | 4 | (8.2) | 1 | (4.2) |
| Respiratory System | ||||
| Respiratory Disorder | 4 | (8.2) | 1 | (4.2) |
| Skin and Appendages | ||||
| Skin Reaction | 6 | (12.2) | 0 | (0.0) |
| Urogenital System | ||||
| Urinary Disorders | 5 | (10.2) | 4 | (16.7) |
In these same studies, the following adverse reactions were reported in less than 5% of the patients on LUPRON DEPOT 30 mg for 4-month administration.
Body As A Whole - Abscess, Accidental injury, Allergic reaction, Cyst, Fever, Generalized edema, Hernia, Neck pain, Neoplasm
Cardiovascular System - Atrial fibrillation, Deep thrombophlebitis, Hypertension
Digestive System - Anorexia, Eructation, Gastrointestinal hemorrhage, Gingivitis, Gum hemorrhage, Hepatomegaly, Increased appetite, Intestinal obstruction, Periodontal abscess
Hemic and Lymphatic System - Lymphadenopathy
Metabolic and Nutritional Disorders - Healing abnormal, Hypoxia, Weight loss
Musculoskeletal System - Leg cramps, Pathological fracture, Ptosis
Nervous System - Abnormal thinking, Amnesia, Confusion, Convulsion, Dementia, Depression, Insomnia/sleep disorders, Libido decreased*, Neuropathy, Paralysis
Respiratory System - Asthma, Bronchitis, Hiccup, Lung disorder, Sinusitis, Voice alteration
Skin and Appendages - Herpes zoster, Melanosis
Urogenital System - Bladder carcinoma, Epididymitis, Impotence*, Prostate disorder, Testicular atrophy*, Urinary incontinence, Urinary tract infection.
* Physiologic effect of decreased testosterone.
Laboratory Abnormalities
Abnormalities of certain parameters were observed, but their relationship to drug treatment is difficult to assess in this population. The following were recorded in ≥ 5% of patients: Decreased bicarbonate, Decreased hemoglobin/hematocrit/RBC, Hyperlipidemia (total cholesterol, LDL-cholesterol, triglycerides), Decreased HDL-cholesterol, Eosinophilia, Increased glucose, Increased liver function tests (ALT, AST, GGTP, LDH), Increased phosphorus. Additional laboratory abnormalities were reported: Increased BUN and PT, Leukopenia, Thrombocytopenia, Uricaciduria.
6.4 LUPRON DEPOT 45 mg for 6-Month Administration
One open label, multicenter study was conducted with LUPRON DEPOT 45 mg for 6-month administration in 151 prostate cancer patients. Patients were treated for 48 weeks, with 139/151 receiving two injections 24 weeks apart.
In the above described clinical trial, the following adverse events were reported in ≥ 5% of the patients during the treatment period. The Table 5 includes all adverse events reported in ≥ 5% of patients as well as the incidences of these adverse events that were considered, by the treating physician, to have a definite or possible relationship to LUPRON.
| Table 5. Adverse Events in ≥ 5% of Patients | ||||
| LUPRON DEPOT 45 mg for 6-Month Administration | ||||
| Treatment Emergent | Treatment Related | |||
| Adverse Event | N = 151 | (%) | N = 151 | (%) |
| Hot Flush/Flushing | 89 | 58.9 | 88 | 58.3 |
| Injection Site Pain/Discomfort | 29 | 19.2 | 16 | 10.6 |
| Upper Respiratory Tract Infection/Influenza-like Illness1 | 32 | 21.2 | 0 | 0 |
| Fatigue/Lethargy | 20 | 13.2 | 18 | 11.9 |
| Constipation | 15 | 9.9 | 5 | 3.3 |
| Arthralgia | 14 | 9.3 | 2 | 1.3 |
| Insomnia/Sleep Disorder | 13 | 8.6 | 5 | 3.3 |
| Headache/Sinus Headache | 12 | 7.9 | 3 | 2.0 |
| Musculoskeletal Pain/ Myalgia | 12 | 7.9 | 3 | 2.0 |
| Second Primary Neoplasm2 | 11 | 7.3 | 0 | 0 |
| Cough | 10 | 6.6 | 2 | 1.3 |
| Hematuria/Hemorrhagic Cystitis | 10 | 6.6 | 0 | 0 |
| Hypertension/BP Increased | 10 | 6.6 | 3 | 2.0 |
| Rash | 9 | 6.0 | 3 | 2.0 |
| Dysuria | 9 | 6.0 | 1 | 0.7 |
| Urinary Tract Infection/Cystitis | 9 | 6.0 | 0 | 0 |
| Anemia/Hemoglobin Decreased | 10 | 6.6 | 2 | 1.3 |
| Back Pain | 8 | 5.3 | 0 | 0 |
| COPD | 8 | 5.3 | 0 | 0 |
| Dizziness | 8 | 5.3 | 3 | 2.0 |
| Dyspnea/Dyspnea on Exertion | 8 | 5.3 | 2 | 1.3 |
| Nocturia | 8 | 5.3 | 2 | 1.3 |
| Peripheral/Pitting Edema | 8 | 5.3 | 2 | 1.3 |
| Coronary Artery Disease/Angina | 8 | 5.3 | 1 | 0.7 |
1Includes influenza, nasal congestion, nasopharyngitis, rhinorrhea, upper respiratory tract infection, and viral upper respiratory tract infection
2Includes basal cell carcinoma, bladder transitional cell carcinoma, lung neoplasm, malignant melanoma, non-Hodgkin’s lymphoma, and squamous cell carcinoma
The following adverse events led to discontinuation; fatigue, hot flush, second primary neoplasm, asthenia, coronary artery disease, constipation, hyperkalemia, and sleep disorder. Serious adverse events in ≥ 2% of patients, regardless of causality, included chronic obstructive pulmonary disease, coronary artery disease/angina, cerebrovascular accident/transient ischemic attack, pneumonia, and second primary neoplasms.
Laboratory Abnormalities
At baseline, 13.9% of patients had a CTCAE v4.0 grade 1 or 2 decreased hemoglobin. During the study, 42.4% of subjects had grade 1 decreased hemoglobin (10 - <12-5 g/dL), 2.0% had grade 2 (8 - <10 g/dL) and 1.3% of subjects had grade 3 or 4 (<8 g/dL). Likewise, 28.5% of patients had a grade 1 or 2 increased cholesterol at baseline while 55.0% had grade 1 increased cholesterol (>199- 300 mg/dL), 3.3% had a grade 2 increase (>300-400 mg/dL), and 0.7% of subjects had grade 3 (>400 mg/dL) during the study.
6.5 Postmarketing
The following adverse reactions have been identified during post-approval use of LUPRON DEPOT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
During postmarketing surveillance, which includes other dosage forms and other patient populations, the following adverse reactions were reported.
Like other drugs in this class, mood swings, including depression, have been reported. There have been very rare reports of suicidal ideation and attempt. Many, but not all, of these patients had a history of depression or other psychiatric illness. Patients should be counseled on the possibility of development or worsening of depression during treatment with LUPRON.
Symptoms consistent with an anaphylactoid or asthmatic process have been rarely (incidence rate of about 0.002%) reported. Rash, urticaria, and photosensitivity reactions have also been reported.
Changes in Bone Density - Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with a GnRH agonist analog. In a clinical trial, 25 men with prostate cancer, 12 of whom had been treated previously with leuprolide acetate for at least six months, underwent bone density studies as a result of pain. The leuprolide-treated group had lower bone density scores than the nontreated control group. It can be anticipated that long periods of medical castration in men will have effects on bone density.
Pituitary apoplexy - During post-marketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed, with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
Localized reactions including induration and abscess have been reported at the site of injection.
Symptoms consistent with fibromyalgia (e.g., joint and muscle pain, headaches, sleep disorders, gastrointestinal distress, and shortness of breath) have been reported individually and collectively.
Cardiovascular System - Hypotension, Myocardial infarction, Pulmonary embolism
Respiratory, thoracic and mediastinal disorder - Interstitial lung disease
Hepato-biliary disorder - Serious drug-induced liver injury
Hemic and Lymphatic System - Decreased WBC
Central/Peripheral Nervous System - Convulsion, Peripheral neuropathy, Spinal fracture/paralysis
Endocrine System - Diabetes
Musculoskeletal System - Tenosynovitis-like symptoms
Urogenital System - Prostate pain
See other LUPRON DEPOT and LUPRON Injection package inserts for other reactions reported in women and pediatric populations.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on findings in animal studies and mechanism of action, LUPRON DEPOT may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data in pregnant women to inform the drug-associated risk. In animal developmental and reproductive toxicology studies, administration of a monthly formulation of leuprolide acetate on day 6 of pregnancy (sustained exposure was expected throughout the period of organogenesis) caused adverse embryo-fetal toxicity in animals at doses less than the human dose based on body surface area using an estimated daily dose (see data). Advise pregnant patients and females of reproductive potential of the potential risk to the fetus.
Data
Animal Data
Major fetal malformations were observed in developmental and reproductive toxicology studies in rabbits after a single administration of the monthly formulation of leuprolide acetate on day 6 of pregnancy at doses of 0.00024, 0.0024, and 0.024 mg/kg (approximately 1/1600 to 1/16 the human dose based on body surface area using an estimated daily dose in animals and humans). Since a depot formulation was utilized in the study, a sustained exposure to leuprolide was expected throughout the period of organogenesis and to the end of gestation. Similar studies in rats did not demonstrate an increase in fetal malformations, however, there was increased fetal mortality and decreased fetal weights with the two higher doses of the monthly formulation of leuprolide acetate in rabbits and with the highest dose (0.024 mg/kg) in rats.
12. Lupron Depot - Clinical Pharmacology
12.1 Mechanism of Action
Leuprolide acetate, a GnRH agonist, acts as an inhibitor of gonadotropin secretion. Animal studies indicate that following an initial stimulation, continuous administration of leuprolide acetate results in suppression of ovarian and testicular steroidogenesis. This effect was reversible upon discontinuation of drug therapy.
Administration of leuprolide acetate has resulted in inhibition of the growth of certain hormone dependent tumors (prostatic tumors in Noble and Dunning male rats and DMBA-induced mammary tumors in female rats) as well as atrophy of the reproductive organs.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted with leuprolide acetate in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet-cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no leuprolide acetate-induced tumors or pituitary abnormalities were observed at a dose as high as 60 mg/kg for two years. Patients have been treated with leuprolide acetate for up to three years with doses as high as 10 mg/day and for two years with doses as high as 20 mg/day without demonstrable pituitary abnormalities.
Genotoxicity studies were conducted with leuprolide acetate using bacterial and mammalian systems. These studies provided no evidence of mutagenic effects or chromosomal aberrations.
Leuprolide may reduce male and female fertility. Administration of leuprolide acetate to male and female rats at dose of 0.024, 0.24, and 2.4 mg/kg as monthly depot formulation for up to 3 months (approximately as low as 1/30 of the human dose based on body surface area using an estimated daily dose in animals and humans) caused atrophy of the reproductive organs, and suppression of reproductive function. These changes were reversible upon cessation of treatment.
| LUPRON DEPOT
leuprolide acetate kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LUPRON DEPOT
leuprolide acetate kit |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| LUPRON DEPOT
leuprolide acetate kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LUPRON DEPOT
leuprolide acetate kit |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - AbbVie Inc. (078458370) |