Drug Detail:Hydroquinone (monograph) (Epiquin micro)
Drug Class:
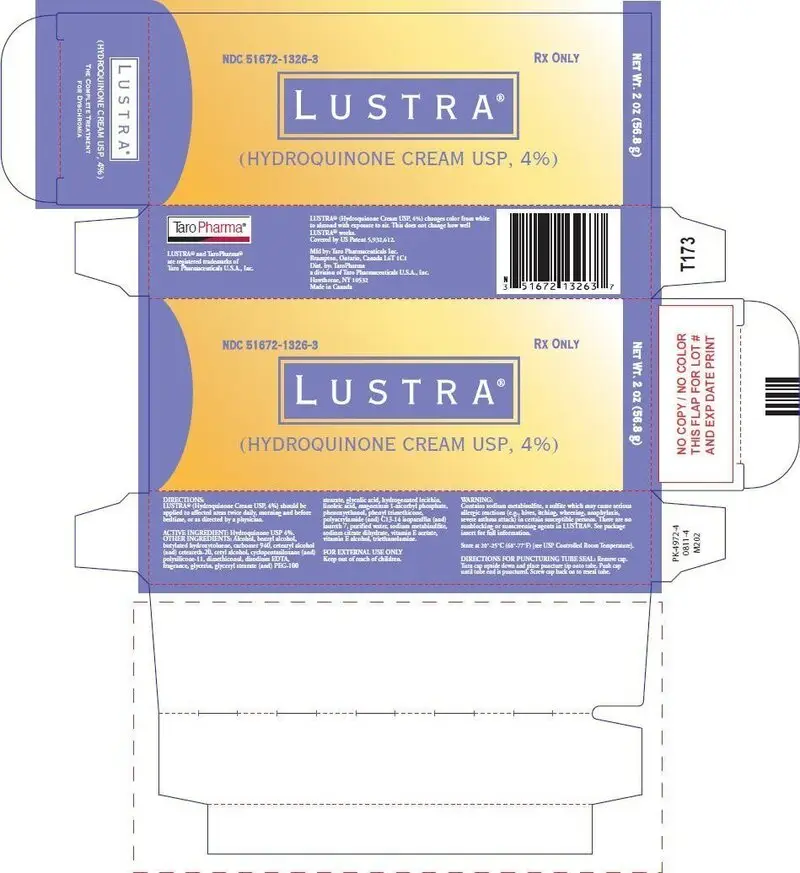
Lustra Cream Description
Hydroquinone is 1,4-benzenediol. Hydroquinone is structurally related to monobenzone. Hydroquinone occurs as fine, white needles. The drug is freely soluble in water and in alcohol and has a pKa of 9.96. Chemically, hydroquinone is designated as p-dihydroxybenzene; the empirical formula is C6H6O2; molecular weight 110.1. The structural formula is:
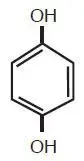
C6H6O2
Warnings
- A.
- CAUTION: Hydroquinone is a depigmenting agent which may produce unwanted cosmetic effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this medication.
- B.
- Test for skin sensitivity before using LUSTRA® or LUSTRA-AF® by applying a small amount to an unbroken patch of skin and check within 24 hours. Minor redness is not a contraindication, but where there is itching, vesicle formation, or excessive inflammatory response further treatment is not advised. Close patient supervision is recommended. Contact with the eyes should be avoided.
If no lightening effect is noted after two months of treatment, use of LUSTRA® or LUSTRA-AF® should be discontinued. LUSTRA-AF® is formulated for use as a treatment for dyschromia and should not be used for the prevention of sunburn.
- C.
- Sunscreen use is an essential aspect of hydroquinone therapy, because even minimal sunlight sustains melanocytic activity. During treatment and maintenance therapy, sun exposure should be avoided on treated skin by application of a broad spectrum sunscreen (SPF 15 or greater) or by use of protective clothing to prevent repigmentation. Although LUSTRA® has an antioxidant system in its vehicle, there are no sunblocking or sunscreening agents in LUSTRA®. The sunscreens in LUSTRA-AF® provide the necessary sun protection during therapy. During and after the use of LUSTRA-AF®, sun exposure should be limited or sun-protective clothing should be used to cover the treated areas to prevent repigmentation.
- D.
- Keep this and all medications out of the reach of children. In case of accidental ingestion, contact a physician or a poison control center immediately.
- E.
- WARNING: Contains sodium metabisulfite, a sulfite which may cause serious allergic reactions (e.g., hives, itching, wheezing, anaphylaxis, severe asthma attack) in certain susceptible persons.
- F.
- On rare occasions, a gradual blue-black darkening of the skin may occur. In which case, use of LUSTRA® or LUSTRA-AF® should be discontinued and a physician contacted immediately.
| LUSTRA
hydroquinone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
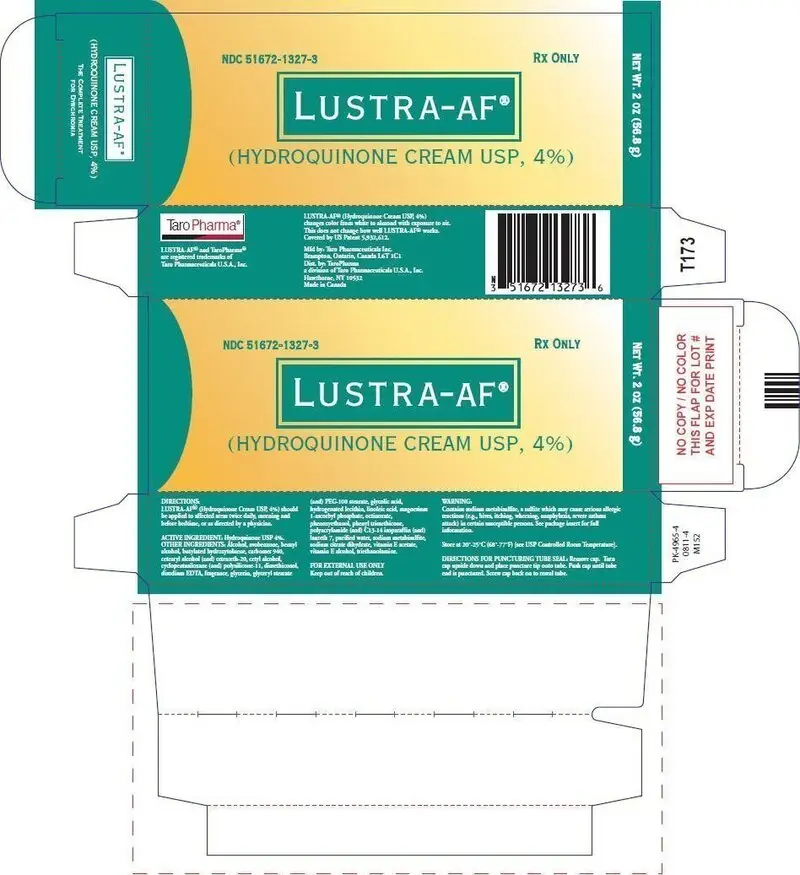
| LUSTRA-AF
hydroquinone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceuticals Inc. | 206263295 | MANUFACTURE(51672-1326, 51672-1327) | |