Drug Detail:Lyrica (Pregabalin [ pre-gab-a-lin ])
Drug Class: Gamma-aminobutyric acid analogs
Highlights of Prescribing Information
LYRICA (pregabalin) Capsules, CV
LYRICA (pregabalin) Oral Solution, CV
Initial U.S. Approval: 2004
Recent Major Changes
| Warnings and Precautions, Respiratory Depression (5.4) | 4/2020 |
Indications and Usage for Lyrica
LYRICA is indicated for:
- Neuropathic pain associated with diabetic peripheral neuropathy (DPN) (1)
- Postherpetic neuralgia (PHN) (1)
- Adjunctive therapy for the treatment of partial-onset seizures in patients 1 month of age and older (1)
- Fibromyalgia (1)
- Neuropathic pain associated with spinal cord injury (1)
Lyrica Dosage and Administration
- For adult indications, begin dosing at 150 mg/day. For partial-onset seizure dosing in pediatric patients 1 month of age and older, refer to section 2.4. (2.2, 2.3, 2.4, 2.5, 2.6)
- Dosing recommendations:
| INDICATION | Dosing Regimen | Maximum Dose |
|---|---|---|
| DPN Pain (2.2) | 3 divided doses per day | 300 mg/day within 1 week |
| PHN (2.3) | 2 or 3 divided doses per day | 300 mg/day within 1 week. Maximum dose of 600 mg/day. |
| Adjunctive Therapy for Partial-Onset Seizures in Pediatric and Adult Patients Weighing 30 kg or More (2.4) | 2 or 3 divided doses per day | Maximum dose of 600 mg/day. |
| Adjunctive Therapy for Partial-Onset Seizures in Pediatric Patients Weighing Less than 30 kg (2.4) | 1 month to less than 4 years:
3 divided doses per day 4 years and older: 2 or 3 divided doses per day | 14 mg/kg/day. |
| Fibromyalgia (2.5) | 2 divided doses per day | 300 mg/day within 1 week. Maximum dose of 450 mg/day. |
| Neuropathic Pain Associated with Spinal Cord Injury (2.6) | 2 divided doses per day | 300 mg/day within 1 week. Maximum dose of 600 mg/day. |
- Dose should be adjusted in adult patients with reduced renal function. (2.7)
Dosage Forms and Strengths
- Capsules: 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg, and 300 mg. (3)
- Oral Solution: 20 mg/mL. (3)
Contraindications
- Known hypersensitivity to pregabalin or any of its components. (4)
Warnings and Precautions
- Angioedema (e.g., swelling of the throat, head and neck) can occur, and may be associated with life-threatening respiratory compromise requiring emergency treatment. Discontinue LYRICA immediately in these cases. (5.1)
- Hypersensitivity reactions (e.g., hives, dyspnea, and wheezing) can occur. Discontinue LYRICA immediately in these patients. (5.2)
- Antiepileptic drugs, including LYRICA, increase the risk of suicidal thoughts or behavior. (5.3)
- Respiratory depression: May occur with LYRICA, when used with concomitant CNS depressants or in the setting of underlying respiratory impairment. Monitor patients and adjust dosage as appropriate. (5.4)
- LYRICA may cause dizziness and somnolence and impair patients' ability to drive or operate machinery. (5.5)
- Increased seizure frequency or other adverse reactions may occur if LYRICA is rapidly discontinued. Withdraw LYRICA gradually over a minimum of 1 week. (5.6)
- LYRICA may cause peripheral edema. Exercise caution when co-administering LYRICA and thiazolidinedione antidiabetic agents. (5.7)
Adverse Reactions/Side Effects
Most common adverse reactions (greater than or equal to 5% and twice placebo) in adults are dizziness, somnolence, dry mouth, edema, blurred vision, weight gain, and thinking abnormal (primarily difficulty with concentration/attention). (6.1)
Most common adverse reactions (greater than or equal to 5% and twice placebo) in pediatric patients for the treatment of partial-onset seizures are increased weight and increased appetite. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Pregnancy: May cause fetal harm. Advise of potential risk to the fetus. (8.1)
- Lactation: Breastfeeding is not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2020
Full Prescribing Information
1. Indications and Usage for Lyrica
LYRICA is indicated for:
- Management of neuropathic pain associated with diabetic peripheral neuropathy
- Management of postherpetic neuralgia
- Adjunctive therapy for the treatment of partial-onset seizures in patients 1 month of age and older
- Management of fibromyalgia
- Management of neuropathic pain associated with spinal cord injury
2. Lyrica Dosage and Administration
2.1 Important Administration Instructions
LYRICA is given orally with or without food.
When discontinuing LYRICA, taper gradually over a minimum of 1 week [see Warnings and Precautions (5.6)].
Because LYRICA is eliminated primarily by renal excretion, adjust the dose in adult patients with reduced renal function [see Dosage and Administration (2.7)].
2.2 Neuropathic Pain Associated with Diabetic Peripheral Neuropathy in Adults
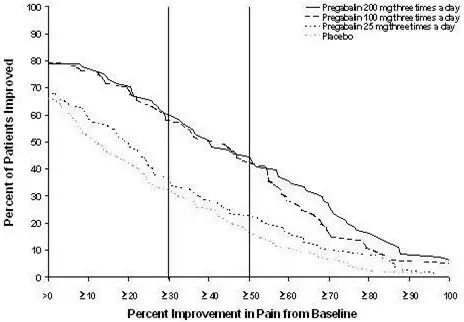
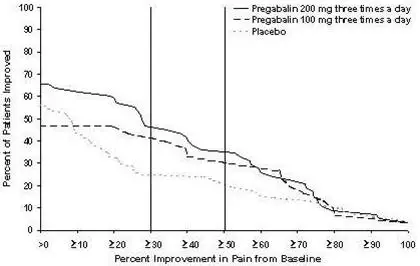
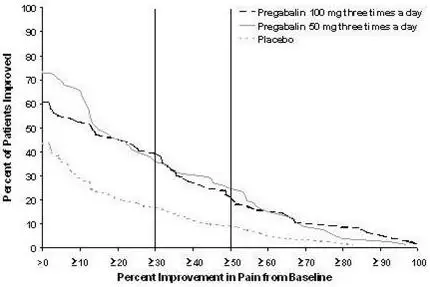
The maximum recommended dose of LYRICA is 100 mg three times a day (300 mg/day) in patients with creatinine clearance of at least 60 mL/min. Begin dosing at 50 mg three times a day (150 mg/day). The dose may be increased to 300 mg/day within 1 week based on efficacy and tolerability.
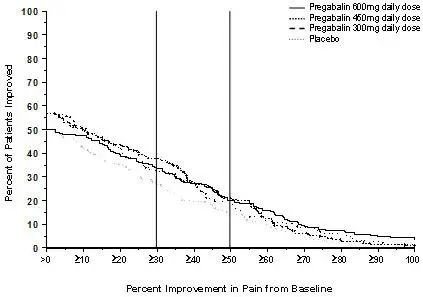
Although LYRICA was also studied at 600 mg/day, there is no evidence that this dose confers additional significant benefit and this dose was less well tolerated. In view of the dose-dependent adverse reactions, treatment with doses above 300 mg/day is not recommended [see Adverse Reactions (6.1)].
2.3 Postherpetic Neuralgia in Adults
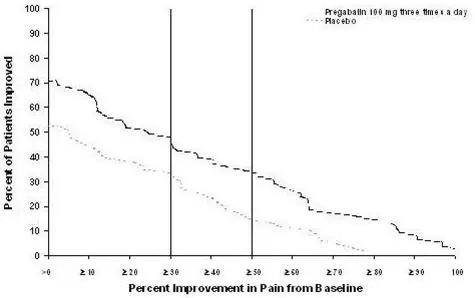
The recommended dose of LYRICA is 75 to 150 mg two times a day, or 50 to 100 mg three times a day (150 to 300 mg/day) in patients with creatinine clearance of at least 60 mL/min. Begin dosing at 75 mg two times a day, or 50 mg three times a day (150 mg/day). The dose may be increased to 300 mg/day within 1 week based on efficacy and tolerability.
Patients who do not experience sufficient pain relief following 2 to 4 weeks of treatment with 300 mg/day, and who are able to tolerate LYRICA, may be treated with up to 300 mg two times a day, or 200 mg three times a day (600 mg/day). In view of the dose-dependent adverse reactions and the higher rate of treatment discontinuation due to adverse reactions, reserve dosing above 300 mg/day for those patients who have on-going pain and are tolerating 300 mg daily [see Adverse Reactions (6.1)].
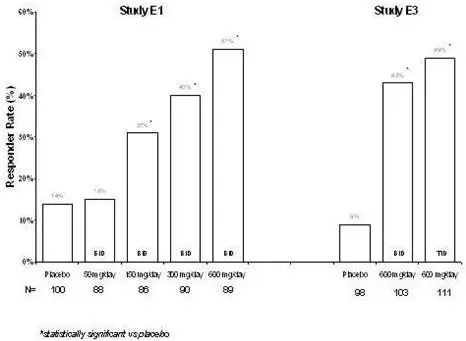
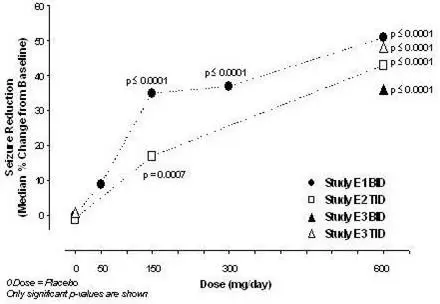
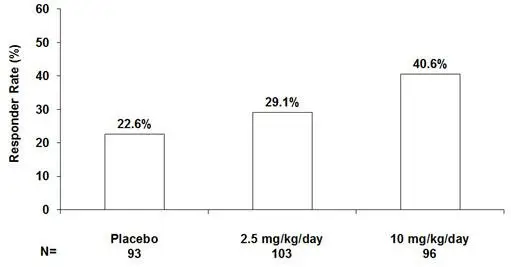
2.4 Adjunctive Therapy for Partial-Onset Seizures in Patients 1 Month of Age and Older
The recommended dosages for adults and pediatric patients 1 month of age and older are included in Table 1. Administer the total daily dosage orally in two or three divided doses as indicated in Table 1. In pediatric patients, the recommended dosing regimen is dependent upon body weight. Based on clinical response and tolerability, dosage may be increased, approximately weekly.
| Age and Body Weight | Recommended Initial Dosage | Recommended Maximum Dosage | Frequency of Administration |
|---|---|---|---|
| Adults (17 years and older) | 150 mg/day | 600 mg/day | 2 or 3 divided doses |
| Pediatric patients weighing 30 kg or more | 2.5 mg/kg/day | 10 mg/kg/day (not to exceed 600 mg/day) | 2 or 3 divided doses |
| Pediatric patients weighing less than 30 kg | 3.5 mg/kg/day | 14 mg/kg/day | 1 month to less than 4 years of age:
3 divided doses 4 years of age and older: 2 or 3 divided doses |
Both the efficacy and adverse event profiles of LYRICA have been shown to be dose-related.
The effect of dose escalation rate on the tolerability of LYRICA has not been formally studied.
The efficacy of adjunctive LYRICA in patients taking gabapentin has not been evaluated in controlled trials. Consequently, dosing recommendations for the use of LYRICA with gabapentin cannot be offered.
2.5 Management of Fibromyalgia in Adults
The recommended dose of LYRICA for fibromyalgia is 300 to 450 mg/day. Begin dosing at 75 mg two times a day (150 mg/day). The dose may be increased to 150 mg two times a day (300 mg/day) within 1 week based on efficacy and tolerability. Patients who do not experience sufficient benefit with 300 mg/day may be further increased to 225 mg two times a day (450 mg/day). Although LYRICA was also studied at 600 mg/day, there is no evidence that this dose confers additional benefit and this dose was less well tolerated. In view of the dose-dependent adverse reactions, treatment with doses above 450 mg/day is not recommended [see Adverse Reactions (6.1)].
2.6 Neuropathic Pain Associated with Spinal Cord Injury in Adults
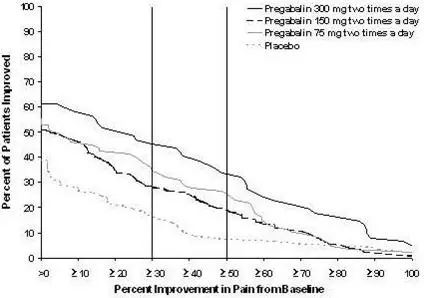
The recommended dose range of LYRICA for the treatment of neuropathic pain associated with spinal cord injury is 150 to 600 mg/day. The recommended starting dose is 75 mg two times a day (150 mg/day). The dose may be increased to 150 mg two times a day (300 mg/day) within 1 week based on efficacy and tolerability. Patients who do not experience sufficient pain relief after 2 to 3 weeks of treatment with 150 mg two times a day and who tolerate LYRICA may be treated with up to 300 mg two times a day [see Clinical Studies (14.5)].
2.7 Dosing for Adult Patients with Renal Impairment
In view of dose-dependent adverse reactions and since LYRICA is eliminated primarily by renal excretion, adjust the dose in adult patients with reduced renal function. The use of LYRICA in pediatric patients with compromised renal function has not been studied.
Base the dose adjustment in patients with renal impairment on creatinine clearance (CLcr), as indicated in Table 2. To use this dosing table, an estimate of the patient's CLcr in mL/min is needed. CLcr in mL/min may be estimated from serum creatinine (mg/dL) determination using the Cockcroft and Gault equation:
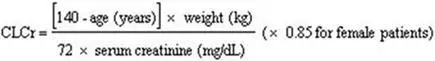
Next, refer to the Dosage and Administration section to determine the recommended total daily dose based on indication, for a patient with normal renal function (CLcr greater than or equal to 60 mL/min). Then refer to Table 2 to determine the corresponding renal adjusted dose.
(For example: A patient initiating LYRICA therapy for postherpetic neuralgia with normal renal function (CLcr greater than or equal to 60 mL/min), receives a total daily dose of 150 mg/day pregabalin. Therefore, a renal impaired patient with a CLcr of 50 mL/min would receive a total daily dose of 75 mg/day pregabalin administered in two or three divided doses.)
For patients undergoing hemodialysis, adjust the pregabalin daily dose based on renal function. In addition to the daily dose adjustment, administer a supplemental dose immediately following every 4-hour hemodialysis treatment (see Table 2).
| Creatinine Clearance (CLcr) (mL/min) | Total Pregabalin Daily Dose (mg/day)* | Dose Regimen | |||
|---|---|---|---|---|---|
| TID= Three divided doses; BID = Two divided doses; QD = Single daily dose. | |||||
|
|||||
| Greater than or equal to 60 | 150 | 300 | 450 | 600 | BID or TID |
| 30–60 | 75 | 150 | 225 | 300 | BID or TID |
| 15–30 | 25–50 | 75 | 100–150 | 150 | QD or BID |
| Less than 15 | 25 | 25–50 | 50–75 | 75 | QD |
| Supplementary dosage following hemodialysis (mg)† | |||||
| Patients on the 25 mg QD regimen: take one supplemental dose of 25 mg or 50 mg | |||||
| Patients on the 25–50 mg QD regimen: take one supplemental dose of 50 mg or 75 mg | |||||
| Patients on the 50–75 mg QD regimen: take one supplemental dose of 75 mg or 100 mg | |||||
| Patients on the 75 mg QD regimen: take one supplemental dose of 100 mg or 150 mg | |||||
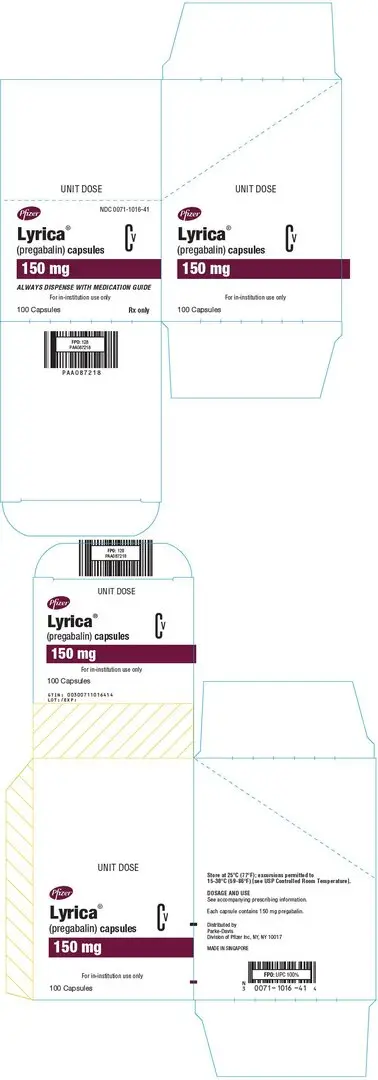
3. Dosage Forms and Strengths
Capsules: 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg, and 300 mg
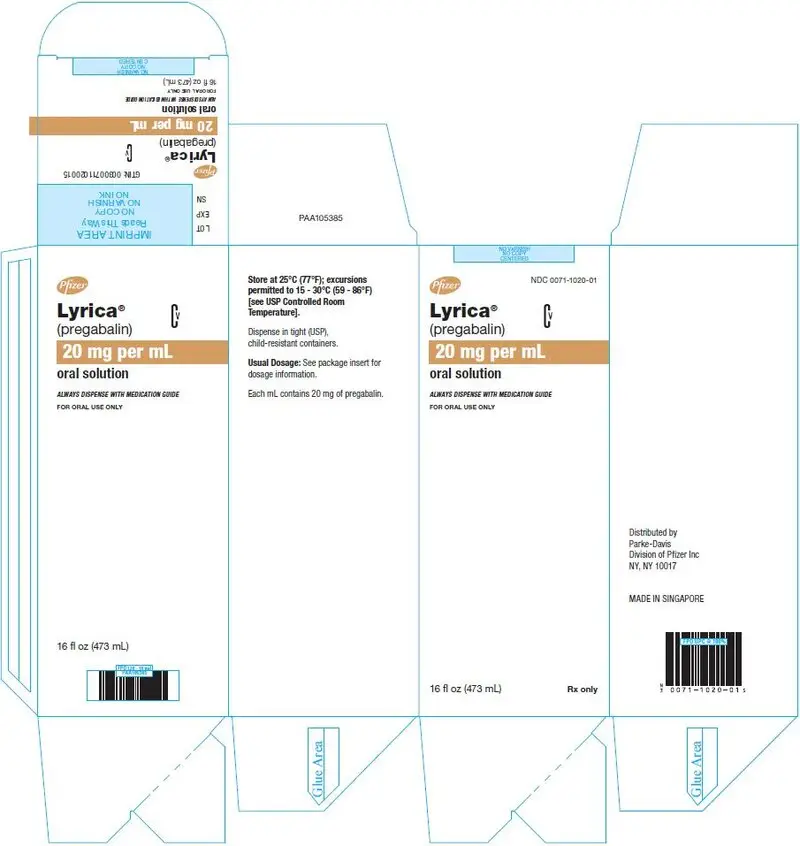
Oral Solution: 20 mg/mL
[see Description (11) and How Supplied/Storage and Handling (16)]
4. Contraindications
LYRICA is contraindicated in patients with known hypersensitivity to pregabalin or any of its components. Angioedema and hypersensitivity reactions have occurred in patients receiving pregabalin therapy [see Warnings and Precautions (5.2)].
5. Warnings and Precautions
5.1 Angioedema
There have been postmarketing reports of angioedema in patients during initial and chronic treatment with LYRICA. Specific symptoms included swelling of the face, mouth (tongue, lips, and gums), and neck (throat and larynx). There were reports of life-threatening angioedema with respiratory compromise requiring emergency treatment. Discontinue LYRICA immediately in patients with these symptoms.
Exercise caution when prescribing LYRICA to patients who have had a previous episode of angioedema. In addition, patients who are taking other drugs associated with angioedema (e.g., angiotensin converting enzyme inhibitors [ACE-inhibitors]) may be at increased risk of developing angioedema.
5.2 Hypersensitivity
There have been postmarketing reports of hypersensitivity in patients shortly after initiation of treatment with LYRICA. Adverse reactions included skin redness, blisters, hives, rash, dyspnea, and wheezing. Discontinue LYRICA immediately in patients with these symptoms.
5.3 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including LYRICA, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Monitor patients treated with any AED for any indication for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5–100 years) in the clinical trials analyzed.
Table 3 shows absolute and relative risk by indication for all evaluated AEDs.
| Indication | Placebo Patients with Events Per 1000 Patients | Drug Patients with Events Per 1000 Patients | Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients | Risk Difference: Additional Drug Patients with Events Per 1000 Patients |
|---|---|---|---|---|
| Epilepsy | 1.0 | 3.4 | 3.5 | 2.4 |
| Psychiatric | 5.7 | 8.5 | 1.5 | 2.9 |
| Other | 1.0 | 1.8 | 1.9 | 0.9 |
| Total | 2.4 | 4.3 | 1.8 | 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing LYRICA or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
5.4 Respiratory Depression
There is evidence from case reports, human studies, and animal studies associating LYRICA with serious, life-threatening, or fatal respiratory depression when co-administered with central nervous system (CNS) depressants, including opioids, or in the setting of underlying respiratory impairment. When the decision is made to co-prescribe LYRICA with another CNS depressant, particularly an opioid, or to prescribe LYRICA to patients with underlying respiratory impairment, monitor patients for symptoms of respiratory depression and sedation, and consider initiating LYRICA at a low dose. The management of respiratory depression may include close observation, supportive measures, and reduction or withdrawal of CNS depressants (including LYRICA).
There is more limited evidence from case reports, animal studies, and human studies associating LYRICA with serious respiratory depression, without co-administered CNS depressants or without underlying respiratory impairment.
5.5 Dizziness and Somnolence
LYRICA may cause dizziness and somnolence. Inform patients that LYRICA-related dizziness and somnolence may impair their ability to perform tasks such as driving or operating machinery [see Patient Counseling Information (17)].
In the LYRICA controlled trials in adult patients, dizziness was experienced by 30% of LYRICA-treated patients compared to 8% of placebo-treated patients; somnolence was experienced by 23% of LYRICA-treated patients compared to 8% of placebo-treated patients. Dizziness and somnolence generally began shortly after the initiation of LYRICA therapy and occurred more frequently at higher doses. Dizziness and somnolence were the adverse reactions most frequently leading to withdrawal (4% each) from controlled studies. In LYRICA-treated patients reporting these adverse reactions in short-term, controlled studies, dizziness persisted until the last dose in 30% and somnolence persisted until the last dose in 42% of patients [see Drug Interactions (7)].
In the LYRICA controlled trials in pediatric patients 4 to less than 17 years of age and 1 month to less than 4 years of age for the treatment of partial-onset seizures, somnolence was reported in 21% and 15% of LYRICA-treated patients compared to 14% and 9% of placebo-treated patients, respectively, and occurred more frequently at higher doses. For patients 1 month to less than 4 years of age, somnolence includes related terms lethargy, sluggishness, and hypersomnia.
5.6 Increased Risk of Adverse Reactions with Abrupt or Rapid Discontinuation
As with all antiepileptic drugs (AEDs), withdraw LYRICA gradually to minimize the potential of increased seizure frequency in patients with seizure disorders.
Following abrupt or rapid discontinuation of LYRICA, some patients reported symptoms including insomnia, nausea, headache, anxiety, hyperhidrosis, and diarrhea.
If LYRICA is discontinued, taper the drug gradually over a minimum of 1 week rather than discontinue the drug abruptly.
5.7 Peripheral Edema
LYRICA treatment may cause peripheral edema. In short-term trials of patients without clinically significant heart or peripheral vascular disease, there was no apparent association between peripheral edema and cardiovascular complications such as hypertension or congestive heart failure. Peripheral edema was not associated with laboratory changes suggestive of deterioration in renal or hepatic function.
In controlled clinical trials in adult patients, the incidence of peripheral edema was 6% in the LYRICA group compared with 2% in the placebo group. In controlled clinical trials, 0.5% of LYRICA patients and 0.2% placebo patients withdrew due to peripheral edema.
Higher frequencies of weight gain and peripheral edema were observed in patients taking both LYRICA and a thiazolidinedione antidiabetic agent compared to patients taking either drug alone. The majority of patients using thiazolidinedione antidiabetic agents in the overall safety database were participants in studies of pain associated with diabetic peripheral neuropathy. In this population, peripheral edema was reported in 3% (2/60) of patients who were using thiazolidinedione antidiabetic agents only, 8% (69/859) of patients who were treated with LYRICA only, and 19% (23/120) of patients who were on both LYRICA and thiazolidinedione antidiabetic agents. Similarly, weight gain was reported in 0% (0/60) of patients on thiazolidinediones only; 4% (35/859) of patients on LYRICA only; and 7.5% (9/120) of patients on both drugs.
As the thiazolidinedione class of antidiabetic drugs can cause weight gain and/or fluid retention, possibly exacerbating or leading to heart failure, exercise caution when co-administering LYRICA and these agents.
Because there are limited data on congestive heart failure patients with New York Heart Association (NYHA) Class III or IV cardiac status, exercise caution when using LYRICA in these patients.
5.8 Weight Gain
LYRICA treatment may cause weight gain. In LYRICA controlled clinical trials in adult patients of up to 14 weeks, a gain of 7% or more over baseline weight was observed in 9% of LYRICA-treated patients and 2% of placebo-treated patients. Few patients treated with LYRICA (0.3%) withdrew from controlled trials due to weight gain. LYRICA associated weight gain was related to dose and duration of exposure, but did not appear to be associated with baseline BMI, gender, or age. Weight gain was not limited to patients with edema [see Warnings and Precautions (5.7)].
Although weight gain was not associated with clinically important changes in blood pressure in short-term controlled studies, the long-term cardiovascular effects of LYRICA-associated weight gain are unknown.
Among diabetic patients, LYRICA-treated patients gained an average of 1.6 kg (range: -16 to 16 kg), compared to an average 0.3 kg (range: -10 to 9 kg) weight gain in placebo patients. In a cohort of 333 diabetic patients who received LYRICA for at least 2 years, the average weight gain was 5.2 kg.
While the effects of LYRICA-associated weight gain on glycemic control have not been systematically assessed, in controlled and longer-term open label clinical trials with diabetic patients, LYRICA treatment did not appear to be associated with loss of glycemic control (as measured by HbA1C).
5.9 Tumorigenic Potential
In standard preclinical in vivo lifetime carcinogenicity studies of LYRICA, an unexpectedly high incidence of hemangiosarcoma was identified in two different strains of mice [see Nonclinical Toxicology (13.1)]. The clinical significance of this finding is unknown. Clinical experience during LYRICA's premarketing development provides no direct means to assess its potential for inducing tumors in humans.
In clinical studies across various patient populations, comprising 6396 patient-years of exposure in patients greater than 12 years of age, new or worsening-preexisting tumors were reported in 57 patients. Without knowledge of the background incidence and recurrence in similar populations not treated with LYRICA, it is impossible to know whether the incidence seen in these cohorts is or is not affected by treatment.
5.10 Ophthalmological Effects
In controlled studies in adult patients, a higher proportion of patients treated with LYRICA reported blurred vision (7%) than did patients treated with placebo (2%), which resolved in a majority of cases with continued dosing. Less than 1% of patients discontinued LYRICA treatment due to vision-related events (primarily blurred vision).
Prospectively planned ophthalmologic testing, including visual acuity testing, formal visual field testing and dilated funduscopic examination, was performed in over 3600 patients. In these patients, visual acuity was reduced in 7% of patients treated with LYRICA, and 5% of placebo-treated patients. Visual field changes were detected in 13% of LYRICA-treated, and 12% of placebo-treated patients. Funduscopic changes were observed in 2% of LYRICA-treated and 2% of placebo-treated patients.
Although the clinical significance of the ophthalmologic findings is unknown, inform patients to notify their physician if changes in vision occur. If visual disturbance persists, consider further assessment. Consider more frequent assessment for patients who are already routinely monitored for ocular conditions [see Patient Counseling Information (17)].
5.11 Creatine Kinase Elevations
LYRICA treatment was associated with creatine kinase elevations. Mean changes in creatine kinase from baseline to the maximum value were 60 U/L for LYRICA-treated patients and 28 U/L for the placebo patients. In all controlled trials in adult patients across multiple patient populations, 1.5% of patients on LYRICA and 0.7% of placebo patients had a value of creatine kinase at least three times the upper limit of normal. Three LYRICA-treated subjects had events reported as rhabdomyolysis in premarketing clinical trials. The relationship between these myopathy events and LYRICA is not completely understood because the cases had documented factors that may have caused or contributed to these events. Instruct patients to promptly report unexplained muscle pain, tenderness, or weakness, particularly if these muscle symptoms are accompanied by malaise or fever. Discontinue treatment with LYRICA if myopathy is diagnosed or suspected or if markedly elevated creatine kinase levels occur.
5.12 Decreased Platelet Count
LYRICA treatment was associated with a decrease in platelet count. LYRICA-treated subjects experienced a mean maximal decrease in platelet count of 20 × 103/µL, compared to 11 × 103/µL in placebo patients. In the overall database of controlled trials in adult patients, 2% of placebo patients and 3% of LYRICA patients experienced a potentially clinically significant decrease in platelets, defined as 20% below baseline value and less than 150 × 103/µL. A single LYRICA-treated subject developed severe thrombocytopenia with a platelet count less than 20 × 103/ µL. In randomized controlled trials, LYRICA was not associated with an increase in bleeding-related adverse reactions.
5.13 PR Interval Prolongation
LYRICA treatment was associated with PR interval prolongation. In analyses of clinical trial ECG data in adult patients, the mean PR interval increase was 3–6 msec at LYRICA doses greater than or equal to 300 mg/day. This mean change difference was not associated with an increased risk of PR increase greater than or equal to 25% from baseline, an increased percentage of subjects with on-treatment PR greater than 200 msec, or an increased risk of adverse reactions of second or third degree AV block.
Subgroup analyses did not identify an increased risk of PR prolongation in patients with baseline PR prolongation or in patients taking other PR prolonging medications. However, these analyses cannot be considered definitive because of the limited number of patients in these categories.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in the labeling:
- Angioedema [see Warnings and Precautions (5.1)]
- Hypersensitivity [see Warnings and Precautions (5.2)]
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.3)]
- Respiratory Depression [see Warnings and Precautions (5.4)]
- Dizziness and Somnolence [see Warnings and Precautions (5.5)]
- Increased Risk of Adverse Reactions with Abrupt or Rapid Discontinuation [see Warnings and Precautions (5.6)]
- Peripheral Edema [see Warnings and Precautions (5.7)]
- Weight Gain [see Warnings and Precautions (5.8)]
- Tumorigenic Potential [see Warnings and Precautions (5.9)]
- Ophthalmological Effects [see Warnings and Precautions (5.10)]
- Creatine Kinase Elevations [see Warnings and Precautions (5.11)]
- Decreased Platelet Count [see Warnings and Precautions (5.12)]
- PR Interval Prolongation [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In all controlled and uncontrolled trials across various patient populations during the premarketing development of LYRICA, more than 10,000 patients have received LYRICA. Approximately 5000 patients were treated for 6 months or more, over 3100 patients were treated for 1 year or longer, and over 1400 patients were treated for at least 2 years.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of LYRICA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous System Disorders – Headache
Gastrointestinal Disorders – Nausea, Diarrhea
Reproductive System and Breast Disorders – Gynecomastia, Breast Enlargement
Skin and subcutaneous tissue disorders – Bullous pemphigoid
There are postmarketing reports of life-threatening or fatal respiratory depression in patients taking LYRICA with opioids or other CNS depressants, or in the setting of underlying respiratory impairment.
In addition, there are postmarketing reports of events related to reduced lower gastrointestinal tract function (e.g., intestinal obstruction, paralytic ileus, constipation) when LYRICA was co-administered with medications that have the potential to produce constipation, such as opioid analgesics.
7. Drug Interactions
Since LYRICA is predominantly excreted unchanged in the urine, undergoes negligible metabolism in humans (less than 2% of a dose recovered in urine as metabolites), and does not bind to plasma proteins, its pharmacokinetics are unlikely to be affected by other agents through metabolic interactions or protein binding displacement. In vitro and in vivo studies showed that LYRICA is unlikely to be involved in significant pharmacokinetic drug interactions. Specifically, there are no pharmacokinetic interactions between pregabalin and the following antiepileptic drugs: carbamazepine, valproic acid, lamotrigine, phenytoin, phenobarbital, and topiramate. Important pharmacokinetic interactions would also not be expected to occur between LYRICA and commonly used antiepileptic drugs [see Clinical Pharmacology (12)].
8. Use In Specific Populations
8.2 Lactation
Data
A pharmacokinetic study in ten lactating women, who were at least 12 weeks postpartum, evaluated the concentrations of pregabalin in plasma and breast milk. LYRICA 150 mg oral capsule was given every 12 hours (300 mg daily dose) for a total of four doses. Pregabalin was detected in breast milk at average steady-state concentrations approximately 76% of those in maternal plasma. The estimated average daily infant dose of pregabalin from breast milk (assuming mean milk consumption of 150 mL/kg/day) was 0.31 mg/kg/day, which on a mg/kg basis would be approximately 7% of the maternal dose. The study did not evaluate the effects of LYRICA on milk production. Infants did not receive breast milk obtained during the dosing period, therefore, the effects of LYRICA on the breast fed infant were not evaluated.
8.4 Pediatric Use
8.5 Geriatric Use
In controlled clinical studies of LYRICA in neuropathic pain associated with diabetic peripheral neuropathy, 246 patients were 65 to 74 years of age, and 73 patients were 75 years of age or older.
In controlled clinical studies of LYRICA in neuropathic pain associated with postherpetic neuralgia, 282 patients were 65 to 74 years of age, and 379 patients were 75 years of age or older.
In controlled clinical studies of LYRICA in epilepsy, there were only 10 patients 65 to 74 years of age, and 2 patients who were 75 years of age or older.
No overall differences in safety and efficacy were observed between these patients and younger patients.
In controlled clinical studies of LYRICA in fibromyalgia, 106 patients were 65 years of age or older. Although the adverse reaction profile was similar between the two age groups, the following neurological adverse reactions were more frequent in patients 65 years of age or older: dizziness, vision blurred, balance disorder, tremor, confusional state, coordination abnormal, and lethargy.
LYRICA is known to be substantially excreted by the kidney, and the risk of toxic reactions to LYRICA may be greater in patients with impaired renal function. Because LYRICA is eliminated primarily by renal excretion, adjust the dose for elderly patients with renal impairment [see Dosage and Administration (2.7)].
8.6 Renal Impairment
LYRICA is eliminated primarily by renal excretion and dose adjustment is recommended for adult patients with renal impairment [see Dosage and Administration (2.7) and Clinical Pharmacology (12.3)]. The use of LYRICA in pediatric patients with compromised renal function has not been studied.
9. Drug Abuse and Dependence
9.1 Controlled Substance
LYRICA is a Schedule V controlled substance.
LYRICA is not known to be active at receptor sites associated with drugs of abuse. As with any CNS active drug, carefully evaluate patients for history of drug abuse and observe them for signs of LYRICA misuse or abuse (e.g., development of tolerance, dose escalation, drug-seeking behavior).
9.2 Abuse
In a study of recreational users (N=15) of sedative/hypnotic drugs, including alcohol, LYRICA (450 mg, single dose) received subjective ratings of "good drug effect," "high" and "liking" to a degree that was similar to diazepam (30 mg, single dose). In controlled clinical studies in over 5500 patients, 4 % of LYRICA-treated patients and 1 % of placebo-treated patients overall reported euphoria as an adverse reaction, though in some patient populations studied, this reporting rate was higher and ranged from 1 to 12%.
9.3 Dependence
In clinical studies, following abrupt or rapid discontinuation of LYRICA, some patients reported symptoms including insomnia, nausea, headache or diarrhea [see Warnings and Precautions (5.6)], consistent with physical dependence. In the postmarketing experience, in addition to these reported symptoms there have also been reported cases of anxiety and hyperhidrosis.
11. Lyrica Description
Pregabalin is described chemically as (S)-3-(aminomethyl)-5-methylhexanoic acid. The molecular formula is C8H17NO2 and the molecular weight is 159.23. The chemical structure of pregabalin is:
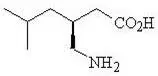
Pregabalin is a white to off-white, crystalline solid with a pKa1 of 4.2 and a pKa2 of 10.6. It is freely soluble in water and both basic and acidic aqueous solutions. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is – 1.35.
LYRICA (pregabalin) Capsules are administered orally and are supplied as imprinted hard-shell capsules containing 25, 50, 75, 100, 150, 200, 225, and 300 mg of pregabalin, along with lactose monohydrate, cornstarch, and talc as inactive ingredients. The capsule shells contain gelatin and titanium dioxide. In addition, the orange capsule shells contain red iron oxide and the white capsule shells contain sodium lauryl sulfate and colloidal silicon dioxide. Colloidal silicon dioxide is a manufacturing aid that may or may not be present in the capsule shells. The imprinting ink contains shellac, black iron oxide, propylene glycol, and potassium hydroxide.
LYRICA (pregabalin) oral solution, 20 mg/mL, is administered orally and is supplied as a clear, colorless solution contained in a 16 fluid ounce white HDPE bottle with a polyethylene-lined closure. The oral solution contains 20 mg/mL of pregabalin, along with methylparaben, propylparaben, monobasic sodium phosphate anhydrous, dibasic sodium phosphate anhydrous, sucralose, artificial strawberry #11545 and purified water as inactive ingredients.
12. Lyrica - Clinical Pharmacology
12.1 Mechanism of Action
LYRICA (pregabalin) binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues. Although the mechanism of action of pregabalin has not been fully elucidated, results with genetically modified mice and with compounds structurally related to pregabalin (such as gabapentin) suggest that binding to the alpha2-delta subunit may be involved in pregabalin's anti-nociceptive and antiseizure effects in animals. In animal models of nerve damage, pregabalin has been shown to reduce calcium-dependent release of pro-nociceptive neurotransmitters in the spinal cord, possibly by disrupting alpha2-delta containing-calcium channel trafficking and/or reducing calcium currents. Evidence from other animal models of nerve damage and persistent pain suggest the anti-nociceptive activities of pregabalin may also be mediated through interactions with descending noradrenergic and serotonergic pathways originating from the brainstem that modulate pain transmission in the spinal cord.
While pregabalin is a structural derivative of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), it does not bind directly to GABAA, GABAB, or benzodiazepine receptors, does not augment GABAA responses in cultured neurons, does not alter rat brain GABA concentration or have acute effects on GABA uptake or degradation. However, in cultured neurons prolonged application of pregabalin increases the density of GABA transporter protein and increases the rate of functional GABA transport. Pregabalin does not block sodium channels, is not active at opiate receptors, and does not alter cyclooxygenase enzyme activity. It is inactive at serotonin and dopamine receptors and does not inhibit dopamine, serotonin, or noradrenaline reuptake.
12.3 Pharmacokinetics
Pregabalin is well absorbed after oral administration, is eliminated largely by renal excretion, and has an elimination half-life of about 6 hours.
13. Nonclinical Toxicology
14. Clinical Studies
14.1 Neuropathic Pain Associated with Diabetic Peripheral Neuropathy
The efficacy of the maximum recommended dose of LYRICA for the management of neuropathic pain associated with diabetic peripheral neuropathy was established in three double-blind, placebo-controlled, multicenter studies with three times a day dosing, two of which studied the maximum recommended dose. Patients were enrolled with either Type 1 or Type 2 diabetes mellitus and a diagnosis of painful distal symmetrical sensorimotor polyneuropathy for 1 to 5 years. A total of 89% of patients completed Studies DPN 1 and DPN 2. The patients had a minimum mean baseline pain score of greater than or equal to 4 on an 11-point numerical pain rating scale ranging from 0 (no pain) to 10 (worst possible pain). The baseline mean pain scores across the two studies ranged from 6.1 to 6.7. Patients were permitted up to 4 grams of acetaminophen per day as needed for pain, in addition to pregabalin. Patients recorded their pain daily in a diary.
14.2 Postherpetic Neuralgia
The efficacy of LYRICA for the management of postherpetic neuralgia was established in three double-blind, placebo-controlled, multicenter studies. These studies enrolled patients with neuralgia persisting for at least 3 months following healing of herpes zoster rash and a minimum baseline score of greater than or equal to 4 on an 11-point numerical pain rating scale ranging from 0 (no pain) to 10 (worst possible pain). Seventy-three percent of patients completed the studies. The baseline mean pain scores across the 3 studies ranged from 6 to 7. Patients were permitted up to 4 grams of acetaminophen per day as needed for pain, in addition to pregabalin. Patients recorded their pain daily in a diary.
14.4 Management of Fibromyalgia
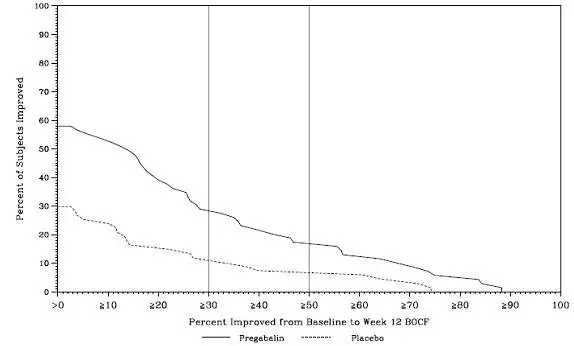
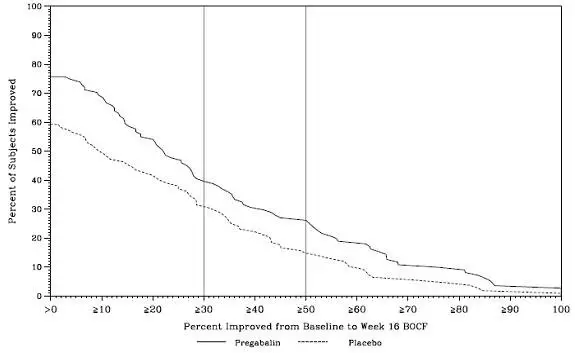
The efficacy of LYRICA for management of fibromyalgia was established in one 14-week, double-blind, placebo-controlled, multicenter study (F1) and one six-month, randomized withdrawal study (F2). Studies F1 and F2 enrolled patients with a diagnosis of fibromyalgia using the American College of Rheumatology (ACR) criteria (history of widespread pain for 3 months, and pain present at 11 or more of the 18 specific tender point sites). The studies showed a reduction in pain by visual analog scale. In addition, improvement was demonstrated based on a patient global assessment (PGIC), and on the Fibromyalgia Impact Questionnaire (FIQ).
14.5 Management of Neuropathic Pain Associated with Spinal Cord Injury
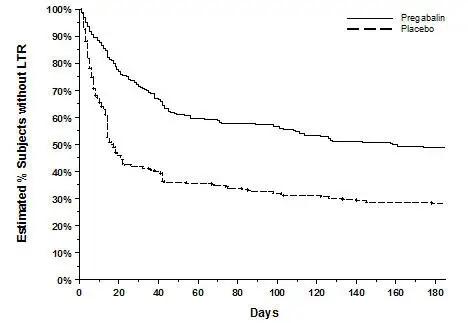
The efficacy of LYRICA for the management of neuropathic pain associated with spinal cord injury was established in two double-blind, placebo-controlled, multicenter studies. Patients were enrolled with neuropathic pain associated with spinal cord injury that persisted continuously for at least three months or with relapses and remissions for at least six months. A total of 63% of patients completed study 1 and 84% completed study 2. The patients had a minimum mean baseline pain score of greater than or equal to 4 on an 11-point numerical pain rating scale ranging from 0 (no pain) to 10 (worst possible pain). The baseline mean pain scores across the two studies ranged from 6.5 to 6.7.
Patients were allowed to take opioids, non-opioid analgesics, antiepileptic drugs, muscle relaxants, and antidepressant drugs if the dose was stable for 30 days prior to screening. Patients were allowed to take acetaminophen and nonsteroidal anti-inflammatory drugs during the studies.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| MEDICATION GUIDE | ||||
|---|---|---|---|---|
| LYRICA (LEER-i-kah) (pregabalin) Capsules, CV | LYRICA (LEER-i-kah) (pregabalin) Oral Solution, CV | |||
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 4/2020 | |||
|
Read this Medication Guide before you start taking LYRICA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. If you have any questions about LYRICA, ask your healthcare provider or pharmacist. |
||||
|
What is the most important information I should know about LYRICA? LYRICA may cause serious side effects including: |
||||
|
|
|||
|
These serious side effects are described below:
|
||||
|
|
|||
|
If you have suicidal thoughts or actions, do not stop LYRICA without first talking to a healthcare provider.
How can I watch for early symptoms of suicidal thoughts and actions?
|
||||
|
||||
|
What is LYRICA? LYRICA is a prescription medicine used in adults, 18 years of age and older to treat:
It is not known if LYRICA is safe and effective in people under 18 years of age for the treatment of fibromyalgia and neuropathic pain with diabetes, shingles, or spinal cord injury. LYRICA is a prescription medicine used in people 1 month of age and older to treat:
For the treatment of partial-onset seizures when taken together with other seizure medicines, it is not known if LYRICA is safe and effective in children under 1 month of age. |
||||
|
Who should not take LYRICA? Do not take LYRICA if you are allergic to pregabalin or any of the ingredients in LYRICA. See "What is the most important information I should know about LYRICA?" for the signs of an allergic reaction. See the end of this Medication Guide for a complete list of ingredients in LYRICA. |
||||
|
What should I tell my healthcare provider before taking LYRICA? Before taking LYRICA, tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins or herbal supplements. LYRICA and other medicines may affect each other causing side effects. Especially tell your healthcare provider if you take:
Know the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist each time you get a new medicine. Do not start a new medicine without talking with your healthcare provider. |
||||
|
How should I take LYRICA?
|
||||
|
What should I avoid while taking LYRICA?
|
||||
|
What are the possible side effects of LYRICA? LYRICA may cause serious side effects, including:
The most common side effects of LYRICA in adults are: |
||||
|
|
|
||
|
The most common side effects of LYRICA in children are weight gain, increase in appetite, and sleepiness. LYRICA caused skin sores in animal studies. Skin sores did not happen in studies in people. If you have diabetes, you should pay attention to your skin while taking LYRICA and tell your healthcare provider about any sores or skin problems. Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of LYRICA. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
|
How should I store LYRICA?
Keep LYRICA and all medicines out of the reach of children. |
||||
|
General information about the safe and effective use of LYRICA Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use LYRICA for a condition for which it was not prescribed. Do not give LYRICA to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about LYRICA that is written for health professionals. |
||||
|
What are the ingredients in LYRICA? Active ingredient: pregabalin Inactive ingredients: LYRICA capsules: lactose monohydrate, cornstarch, talc Capsule shell: gelatin and titanium dioxide; Orange capsule shell: red iron oxide; White capsule shell: sodium lauryl sulfate, colloidal silicon dioxide. Colloidal silicon dioxide is a manufacturing aid that may or may not be present in the capsule shells. Imprinting ink: shellac, black iron oxide, propylene glycol, potassium hydroxide. LYRICA oral solution: methylparaben, propylparaben, monobasic sodium phosphate anhydrous, dibasic sodium phosphate anhydrous, sucralose, artificial strawberry #11545 and purified water.
LAB-0299-17.0 You can also visit the LYRICA website at www.LYRICA.com or call 1-866-459-7422 (1-866-4LYRICA). |
||||
| LYRICA
pregabalin capsule |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LYRICA
pregabalin capsule |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LYRICA
pregabalin capsule |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LYRICA
pregabalin capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LYRICA
pregabalin capsule |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LYRICA
pregabalin capsule |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LYRICA
pregabalin capsule |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LYRICA
pregabalin capsule |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| LYRICA
pregabalin solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Parke-Davis Div of Pfizer Inc (829076962) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Pharmaceuticals LLC | 829084552 | ANALYSIS(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) , LABEL(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) , MANUFACTURE(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) , PACK(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Upjohn Manufacturing Ireland Unlimited Company | 986030667 | ANALYSIS(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) , API MANUFACTURE(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Asia Manufacturing Pte Ltd | 936889401 | ANALYSIS(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) , API MANUFACTURE(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Deutschland GmbH | 341970073 | ANALYSIS(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) , LABEL(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) , MANUFACTURE(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) , PACK(0071-1012, 0071-1013, 0071-1014, 0071-1015, 0071-1016, 0071-1017, 0071-1018, 0071-1019, 0071-1020) | |