Drug Detail:Menactra (Meningococcal conjugate vaccine [ me-nin-je-kok-al-kon-je-gate-vax-een ])
Drug Class: Bacterial vaccines
Highlights of Prescribing Information
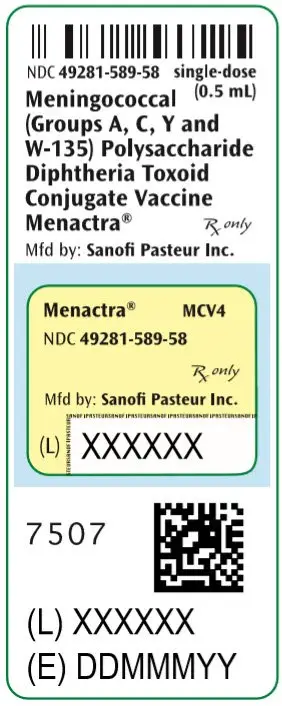
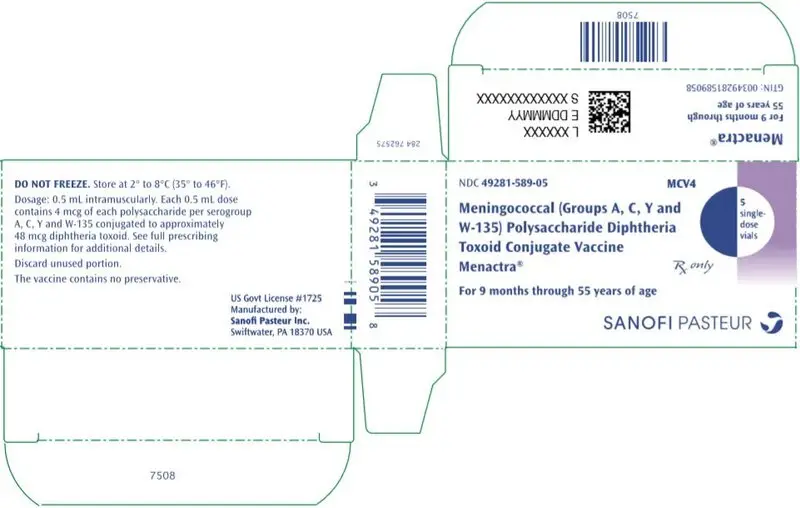
Menactra®, Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine
Solution for Intramuscular Injection
Initial U.S. Approval: 2005
Indications and Usage for Menactra
Menactra is indicated for active immunization to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135. Menactra is approved for use in individuals 9 months through 55 years of age. Menactra does not prevent N meningitidis serogroup B disease. (1)
Menactra Dosage and Administration
A 0.5 mL dose for intramuscular injection. (2)
Primary Vaccination:
- Children 9 through 23 months of age: Two doses, three months apart.
- Individuals 2 through 55 years of age: A single dose.
Booster Vaccination:
- A single booster dose may be given to individuals 15 through 55 years of age at continued risk for meningococcal disease, if at least 4 years have elapsed since the prior dose.
Dosage Forms and Strengths
Solution supplied in 0.5 mL single-dose vials (3)
Contraindications
- Severe allergic reaction (eg, anaphylaxis) after a previous dose of a meningococcal capsular polysaccharide-, diphtheria toxoid- or CRM197-containing vaccine, or to any component of Menactra. (4)
Warnings and Precautions
- Persons previously diagnosed with Guillain-Barré syndrome (GBS) may be at increased risk of GBS following receipt of Menactra. The decision to give Menactra should take into account the potential benefits and risks. (5.1)
Adverse Reactions/Side Effects
- Common (≥10%) solicited adverse events in infants and toddlers 9 and 12 months of age were injection site tenderness, erythema, and swelling; irritability, abnormal crying, drowsiness, appetite loss, vomiting, and fever. (6)
- Common (≥10%) solicited adverse events in individuals 2 through 55 years of age who received a single dose were injection site pain, redness, induration, and swelling; anorexia and diarrhea. Other common solicited adverse events were irritability and drowsiness (2-10 years of age), headache, fatigue, malaise, and arthralgia (11-55 years of age). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc. at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 or http://vaers.hhs.gov.
Drug Interactions
- When Menactra and DAPTACEL® (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed) are to be administered to children 4 through 6 years of age, preference should be given to simultaneous administration of the 2 vaccines or administration of Menactra prior to DAPTACEL. Administration of Menactra one month after DAPTACEL has been shown to reduce meningococcal antibody responses to Menactra. (7.1)
- Pneumococcal antibody responses to some serotypes in Prevnar® (PCV7) were decreased following co-administration of Menactra and PCV7. (7.1)
Use In Specific Populations
- Safety and effectiveness of Menactra have not been established in children younger than 9 months of age, pregnant women, nursing mothers, and adults older than 55 years of age. (8.1, 8.2, 8.4, 8.5)
- A pregnancy registry is available. Contact Sanofi Pasteur Inc. at 1-800-822-2463. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2019
Related/similar drugs
ciprofloxacin, ceftriaxone, Rocephin, rifampin, meningococcal conjugate vaccineFull Prescribing Information
1. Indications and Usage for Menactra
Menactra®, Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine, is indicated for active immunization to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135. Menactra is approved for use in individuals 9 months through 55 years of age. Menactra does not prevent N meningitidis serogroup B disease.
2. Menactra Dosage and Administration
2.1 Preparation for Administration
Menactra is a clear to slightly turbid solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If any of these conditions exist, the vaccine should not be administered.
Withdraw the 0.5 mL dose of vaccine from the single-dose vial using a sterile needle and syringe.
3. Dosage Forms and Strengths
Menactra is a solution supplied in 0.5 mL single-dose vials. [See Description (11) for a complete listing of ingredients.]
4. Contraindications
Severe allergic reaction (eg, anaphylaxis) after a previous dose of a meningococcal capsular polysaccharide-, diphtheria toxoid- or CRM197-containing vaccine, or to any component of Menactra [see Description (11)].
5. Warnings and Precautions
5.1 Guillain-Barré Syndrome
Persons previously diagnosed with Guillain-Barré syndrome (GBS) may be at increased risk of GBS following receipt of Menactra. The decision to give Menactra should take into account the potential benefits and risks.
GBS has been reported in temporal relationship following administration of Menactra (1) (2). The risk of GBS following Menactra vaccination was evaluated in a post-marketing retrospective cohort study [see Post-Marketing Experience (6.2)].
5.2 Preventing and Managing Allergic Vaccine Reactions
Prior to administration, the healthcare provider should review the immunization history for possible vaccine sensitivity and previous vaccination-related adverse reactions to allow an assessment of benefits and risks. Epinephrine and other appropriate agents used for the control of immediate allergic reactions must be immediately available should an acute anaphylactic reaction occur.
5.3 Altered Immunocompetence
-
Reduced Immune Response
Some individuals with altered immunocompetence, including some individuals receiving immunosuppressant therapy, may have reduced immune responses to Menactra. -
Complement Deficiency
Persons with certain complement deficiencies and persons receiving treatment that inhibits terminal complement activation (for example, eculizumab) are at increased risk for invasive disease caused by N meningitidis, including invasive disease caused by serogroups A, C, Y and W-135, even if they develop antibodies following vaccination with Menactra [see Clinical Pharmacology (12)].
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
Booster Vaccination Study
In an open-label trial conducted in the US, 834 individuals were enrolled to receive a single dose of Menactra 4-6 years after a prior dose. The median age of participants was 17.1 years at the time of the booster dose.
6.2 Post-Marketing Experience
In addition to reports in clinical trials, worldwide voluntary adverse events reports received since market introduction of Menactra are listed below. This list includes serious events and/or events which were included based on severity, frequency of reporting or a plausible causal connection to Menactra. Because these events were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to vaccination.
-
Blood and Lymphatic System Disorders
Lymphadenopathy -
Immune System Disorders
Hypersensitivity reactions such as anaphylaxis/anaphylactic reaction, wheezing, difficulty breathing, upper airway swelling, urticaria, erythema, pruritus, hypotension -
Nervous System Disorders
Guillain-Barré syndrome, paraesthesia, vasovagal syncope, dizziness, convulsion, facial palsy, acute disseminated encephalomyelitis, transverse myelitis -
Musculoskeletal and Connective Tissue Disorders
Myalgia -
General Disorders and Administrative Site Conditions
Large injection site reactions, extensive swelling of the injected limb (may be associated with erythema, warmth, tenderness or pain at the injection site).
7. Drug Interactions
7.1 Concomitant Administration with Other Vaccines
Menactra vaccine was concomitantly administered with Typhim Vi® [Typhoid Vi Polysaccharide Vaccine] (Typhoid) and Tetanus and Diphtheria Toxoids Adsorbed, For Adult Use (Td) vaccine, in individuals 18 through 55 and 11 through 17 years of age, respectively. In children 4 through 6 years of age, Menactra was co-administered with DAPTACEL, and in children younger than 2 years of age, Menactra was co-administered with one or more of the following vaccines: PCV7, MMR, V, MMRV, or HepA [see Clinical Studies (14) and Adverse Reactions (6)].
When Menactra and DAPTACEL are to be administered to children 4 through 6 years of age, preference should be given to simultaneous administration of the 2 vaccines or administration of Menactra prior to DAPTACEL. Administration of Menactra one month after DAPTACEL has been shown to reduce meningococcal antibody responses to Menactra. Data are not available to evaluate the immune response to Menactra administered to younger children following DAPTACEL or to Menactra administered to persons <11 years of age following other diphtheria toxoid-containing vaccines [see Clinical Studies (14.3)].
Pneumococcal antibody responses to some serotypes in PCV7 were decreased following co-administration of Menactra and PCV7 [see Concomitant Vaccine Administration (14.3)].
Do not mix Menactra with other vaccines in the same syringe. When Menactra is administered concomitantly with other injectable vaccines, the vaccines should be administered with different syringes and given at separate injection sites.
8. Use In Specific Populations
8.1 Pregnancy
Data
Animal Data
A developmental toxicity study was performed in female mice. The animals were administered 0.1 mL of Menactra (in divided doses) at each of the following time points: 14 days prior to mating, and on Days 6 and 18 of gestation (a single human dose is 0.5 mL). There were no vaccine-related fetal malformations or variations, and no adverse effects on pre-weaning development observed in the study.
8.4 Pediatric Use
Menactra is not approved for use in infants under 9 months of age. Available data show that infants administered three doses of Menactra (at 2, 4, and 6 months of age) had diminished responses to each meningococcal vaccine serogroup compared to older children given two doses at 9 and 12 months of age.
11. Menactra Description
Menactra is a sterile, intramuscularly administered vaccine that contains N meningitidis serogroup A, C, Y and W-135 capsular polysaccharide antigens individually conjugated to diphtheria toxoid protein. N meningitidis A, C, Y and W-135 strains are cultured on Mueller Hinton agar (3) and grown in Watson Scherp (4) media containing casamino acid. The polysaccharides are extracted from the N meningitidis cells and purified by centrifugation, detergent precipitation, alcohol precipitation, solvent extraction and diafiltration. To prepare the polysaccharides for conjugation, they are depolymerized, derivatized, and purified by diafiltration. Diphtheria toxin is derived from Corynebacterium diphtheriae grown in modified culture medium containing hydrolyzed casein (5) and is detoxified using formaldehyde. The diphtheria toxoid protein is purified by ammonium sulfate fractionation and diafiltration. The derivatized polysaccharides are covalently linked to diphtheria toxoid and purified by serial diafiltration. The four meningococcal components, present as individual serogroup-specific glycoconjugates, compose the final formulated vaccine. No preservative or adjuvant is added during manufacture. Each 0.5 mL dose may contain residual amounts of formaldehyde of less than 2.66 mcg (0.000532%), by calculation. Potency of Menactra is determined by quantifying the amount of each polysaccharide antigen that is conjugated to diphtheria toxoid protein and the amount of unconjugated polysaccharide present.
Menactra is manufactured as a sterile, clear to slightly turbid liquid. Each 0.5 mL dose of vaccine is formulated in sodium phosphate buffered isotonic sodium chloride solution to contain 4 mcg each of meningococcal A, C, Y and W-135 polysaccharides conjugated to approximately 48 mcg of diphtheria toxoid protein carrier.
The vial stopper is not made with natural rubber latex.
12. Menactra - Clinical Pharmacology
12.1 Mechanism of Action
The presence of bactericidal anti-capsular meningococcal antibodies has been associated with protection from invasive meningococcal disease (6) (7). Menactra induces the production of bactericidal antibodies specific to the capsular polysaccharides of serogroups A, C, Y and W-135.
14. Clinical Studies
14.1 Efficacy
The serum bactericidal assay (SBA) used to test sera contained an exogenous complement source that was either human (SBA-H) or baby rabbit (SBA-BR). (8)
The response to vaccination following two doses of vaccine administered to children 9 and 12 months of age and following one dose of vaccine administered to children 2 through 10 years of age was evaluated by the proportion of participants having an SBA-H antibody titer of 1:8 or greater, for each serogroup. In individuals 11 through 55 years of age, the response to vaccination with a single dose of vaccine was evaluated by the proportion of participants with a 4-fold or greater increase in bactericidal antibody to each serogroup as measured by SBA-BR. For individuals 2 through 55 years of age, vaccine efficacy after a single dose was inferred from the demonstration of immunologic equivalence to a US-licensed meningococcal polysaccharide vaccine, Menomune – A/C/Y/W-135 vaccine as assessed by SBA.
14.2 Immunogenicity
Children 9 through 12 Months of Age
In a randomized, US, multi-center trial, children received Menactra at 9 months and 12 months of age. The first Menactra dose was administered alone, followed by a second Menactra dose given alone (N=404), or with MMRV (N=302), or with PCV7 (N=422). For all participants, sera were obtained approximately 30 days after last vaccination. There were no substantive differences in demographic characteristics between the vaccine groups. The median age range for administration of the first dose of Menactra was 278-279 days of age.
| Vaccinations administered at 12 months of age following a dose of Menactra at 9 months of age | |||||||
|---|---|---|---|---|---|---|---|
| Menactra | Menactra + MMRV | Menactra + PCV7 | |||||
| (N=272-277)† | (N=177-180)† | (N=264-267)† | |||||
| Serogroup | (95% CI)‡ | (95% CI)‡ | (95% CI)‡ | ||||
|
|||||||
| A | % ≥1:8§ | 95.6 | (92.4; 97.7) | 92.7 | (87.8; 96.0) | 90.5 | (86.3; 93.8) |
| GMT | 54.9 | (46.8; 64.5) | 52.0 | (41.8; 64.7) | 41.0 | (34.6; 48.5) | |
| C | % ≥1:8§ | 100.0 | (98.7; 100.0) | 98.9 | (96.0; 99.9) | 97.8 | (95.2; 99.2) |
| GMT | 141.8 | (123.5; 162.9) | 161.9 | (136.3; 192.3) | 109.5 | (94.1; 127.5) | |
| Y | %≥1:8§ | 96.4 | (93.4; 98.2) | 96.6 | (92.8; 98.8) | 95.1 | (91.8; 97.4) |
| GMT | 52.4 | (45.4; 60.6) | 60.2 | (50.4; 71.7) | 39.9 | (34.4; 46.2) | |
| W-135 | %≥1:8§ | 86.4 | (81.8; 90.3) | 88.2 | (82.5; 92.5) | 81.2 | (76.0; 85.7) |
| GMT | 24.3 | (20.8; 28.3) | 27.9 | (22.7; 34.3) | 17.9 | (15.2; 21.0) | |
Administration of Menactra to children at 12 months and 15 months of age was evaluated in a US study. Prior to the first dose, 33.3% [n=16/48] of participants had an SBA-H titer ≥1:8 to Serogroup A, and 0-2% [n=0-1 of 50-51] to Serogroups C, Y and W-135. After the second dose, percentages of participants with an SBA-H titer ≥1:8 were: 85.2%, Serogroup A [n=46/54]; 100.0%, Serogroup C [n=54/54]; 96.3%, Serogroup Y [n=52/54]; 96.2%, Serogroup W-135 [n=50/52].
Immunogenicity in Children 2 through 10 Years of Age
Of 1408 enrolled children 2 through 10 years of age, immune responses evaluated in a subset of Menactra participants (2 through 3 years of age, n=52; 4-10 years of age, n=84) and Menomune – A/C/Y/W-135 participants (2 through 3 years of age, n=53; 4-10 years of age, n=84) were comparable for all four serogroups (Table 6).
| Ages 2 through 3 Years | Ages 4 through 10 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Menactra | Menomune – A/C/Y/W-135 | Menactra | Menomune – A/C/Y/W-135 | ||||||
| N†=48-52 | N†=50-53 | N†=84 | N†=84 | ||||||
| Serogroup | (95% CI)‡ | (95% CI)‡ | (95% CI)‡ | (95% CI)‡ | |||||
|
|||||||||
| A | % ≥1:8§ | 73 | (59,84) | 64 | (50,77) | 81 | (71,89) | 55 | (44,66) |
| GMT | 10 | (8,13) | 10 | (7,12) | 19 | (14,26) | 7 | (6,9) | |
| C | % ≥1:8§ | 63 | (48,76) | 38 | (25,53) | 79 | (68,87) | 48 | (37,59) |
| GMT | 27 | (14,52) | 11 | (5,21) | 28 | (19,41) | 12 | (7,18) | |
| Y | % ≥1:8§ | 88 | (75,95) | 73 | (59,84) | 99 | (94,100) | 92 | (84,97) |
| GMT | 51 | (31,84) | 18 | (11,27) | 99 | (75,132) | 46 | (33,66) | |
| W-135 | % ≥1:8§ | 63 | (47,76) | 33 | (20,47) | 85 | (75,92) | 79 | (68,87) |
| GMT | 15 | (9,25) | 5 | (3,6) | 24 | (18,33) | 20 | (14,27) | |
In the subset of participants 2 through 3 years of age with undetectable pre-vaccination titers (ie, SBA-H titers <1:4 at Day 0), seroconversion rates (defined as the proportions of participants with SBA-H titers ≥1:8 by Day 28) were similar between the Menactra and Menomune – A/C/Y/W-135 recipients. Menactra participants achieved seroconversion rates of: 57%, Serogroup A (n=12/21); 62%, Serogroup C (n=29/47); 84%, Serogroup Y (n=26/31); 53%, Serogroup W-135 (n=20/38). The seroconversion rates for Menomune – A/C/Y/W-135 recipients were: 55%, Serogroup A (n=16/29); 30%, Serogroup C (n=13/43); 57%, Serogroup Y (n=17/30); 26%, Serogroup W-135 (n=11/43).
In the subset of participants 4 through 10 years of age with undetectable pre-vaccination titers (ie, SBA-H titers <1:4 at Day 0), seroconversion rates (defined as the proportions of participants with SBA-H titers ≥1:8 by Day 28) were similar between the Menactra and Menomune – A/C/Y/W-135 recipients. Menactra participants achieved seroconversion rates of: 69%, Serogroup A (n=11/16); 81%, Serogroup C (n=50/62); 98%, Serogroup Y (n=45/46); 69%, Serogroup W-135 (n=27/39). The seroconversion rates for Menomune – A/C/Y/W-135 recipients were: 48%, Serogroup A (n=10/21); 38%, Serogroup C (n=19/50); 84%, Serogroup Y (n=38/45); 68%, Serogroup W-135 (n=26/38).
Immunogenicity in Adults 18 through 55 Years of Age
Results from the comparative clinical trial conducted in 2554 adults aged 18 through 55 years showed that the immune responses to Menactra and Menomune – A/C/Y/W-135 were similar for all four serogroups (Table 7).
| Ages 11 through 18 Years | Ages 18 through 55 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Menactra | Menomune – A/C/Y/W-135 | Menactra | Menomune – A/C/Y/W-135 | ||||||
| N†=423 | N†=423 | N†=1280 | N†=1098 | ||||||
| Serogroup | (95% CI)‡ | (95% CI)‡ | (95% CI)‡ | (95% CI)‡ | |||||
|
|||||||||
| A | % ≥4-fold rise§ | 92.7 | (89.8, 95.0) | 92.4 | (89.5, 94.8) | 80.5 | (78.2, 82.6) | 84.6 | (82.3, 86.7) |
| GMT | 5483 | (4920, 6111) | 3246 | (2910, 3620) | 3897 | (3647, 4164) | 4114 | (3832, 4417) | |
| C | % ≥4-fold rise§ | 91.7 | (88.7, 94.2) | 88.7 | (85.2, 91.5) | 88.5 | (86.6, 90.2) | 89.7 | (87.8, 91.4) |
| GMT | 1924 | (1662, 2228) | 1639 | (1406, 1911) | 3231 | (2955, 3533) | 3469 | (3148, 3823) | |
| Y | % ≥4-fold rise§ | 81.8 | (77.8, 85.4) | 80.1 | (76.0, 83.8) | 73.5 | (71.0, 75.9) | 79.4 | (76.9, 81.8) |
| GMT | 1322 | (1162, 1505) | 1228 | (1088, 1386) | 1750 | (1597, 1918) | 2449 | (2237, 2680) | |
| W-135 | % ≥4-fold rise§ | 96.7 | (94.5, 98.2) | 95.3 | (92.8, 97.1) | 89.4 | (87.6, 91.0) | 94.4 | (92.8, 95.6) |
| GMT | 1407 | (1232, 1607) | 1545 | (1384, 1725) | 1271 | (1172, 1378) | 1871 | (1723, 2032) | |
In participants with undetectable pre-vaccination titers (ie, SBA-BR titers <1:8 at Day 0), seroconversion rates (defined as the proportions of participants achieving a ≥4-fold rise in SBA-BR titers by Day 28) were similar between the Menactra and Menomune – A/C/Y/W-135 recipients. Menactra participants achieved seroconversion rates of: 100%, Serogroup A (n=156/156); 99%, Serogroup C (n=343/345); 91%, Serogroup Y (n=253/279); 97%, Serogroup W-135 (n=360/373). The seroconversion rates for Menomune – A/C/Y/W-135 recipients were: 99%, Serogroup A (n=143/144); 98%, Serogroup C (n=297/304); 97%, Serogroup Y (n=221/228); 99%, Serogroup W-135 (n=325/328).
14.3 Concomitant Vaccine Administration
DAPTACEL and IPV
In a randomized, parallel group, US multi-center clinical trial conducted in children 4 through 6 years of age, Menactra was administered as follows: 30 days after concomitant DTaP (DAPTACEL®, Sanofi Pasteur Limited) + IPV (IPOL®, Sanofi Pasteur SA) [Group A]; concomitantly with DAPTACEL followed 30 days later by IPV [Group B]; concomitantly with IPV followed 30 days later by DAPTACEL [Group C]. Sera were obtained approximately 30 days after each respective vaccination [see Clinical Trials Experience (6.1)].
When Menactra was administered 30 days after DAPTACEL (and IPV) [Group A], significantly lower SBA-H GMTs to all 4 meningococcal serogroups were observed compared to Menactra (and IPV) administered 30 days prior to DAPTACEL [Group C]. When Menactra was administered concomitantly with DAPTACEL [Group B], SBA-H GMTs to meningococcal serogroups A, C, and W-135 were non-inferior to those observed after Menactra (and IPV) [Group C]. The non-inferiority criterion was marginally missed for meningococcal serogroup Y. Non-inferiority of SBA-H GMTs following concomitant administration of Menactra and DAPTACEL compared to those after concomitant Menactra and IPV was concluded if the upper limit of the 2-sided 95% CI of (GMTGroup C divided by GMTGroup B) computed separately for each of the serogroups was <2.
The respective SBA-H GMTs and proportion (%) of Group A, B, and C study participants achieving an SBA-H titer of ≥1:8 are displayed in Table 8.
| Vaccines administered at Visit 1 and 30 days later at Visit 2 | |||||||
|---|---|---|---|---|---|---|---|
| Visit 1 Visit 2 | Group A DAPTACEL + IPV Menactra | Group B Menactra + DAPTACEL IPV | Group C Menactra + IPV DAPTACEL |
||||
| (N=250)† | (N=238)† | (N=121)† | |||||
| Serogroup | (95% CI)‡ | (95% CI)‡ | (95% CI)‡ | ||||
|
|||||||
| A | % ≥1:8§ | 49.6 | (41.0; 58.3) | 67.2 | (58.4; 75.1) | 64.4 | (54.4; 73.6) |
| GMT | 6.7 | (5.7; 8.0) | 10.8 | (8.7; 13.3) | 10.4 | (8.1; 13.3) | |
| C | % ≥1:8§ | 20.3 | (13.9; 28.0) | 50.4 | (41.5; 59.2) | 50.5 | (40.5; 60.5) |
| GMT | 3.3 | (2.7; 3.9) | 8.1 | (6.3; 10.5) | 7.8 | (5.8; 10.7) | |
| Y | %≥1:8§ | 44.2 | (35.8; 52.9) | 80.2 | (72.3; 86.6) | 88.5 | (80.7; 93.9) |
| GMT | 6.5 | (5.1; 8.2) | 18.1 | (14.2; 22.9) | 26.2 | (20.0; 34.4) | |
| W-135 | %≥1:8§ | 55.1 | (46.4; 63.5) | 87.8 | (80.9; 92.9) | 82.7 | (74.0; 89.4) |
| GMT | 8.4 | (6.7; 10.6) | 22.8 | (18.5; 28.1) | 21.7 | (16.6; 28.4) | |
When Menactra was administered concomitantly with DAPTACEL, antibody responses to three of the pertussis antigens (pertussis toxin, filamentous hemagglutinin, and pertactin) (GMCs), tetanus toxin (% participants with antibody concentrations ≥1.0 IU/mL), and diphtheria toxin (% participants with antibody concentrations ≥1.0 IU/mL) were non-inferior to those observed after DAPTACEL and IPV. The pertussis anti-fimbriae GMCs were marginally lower when Menactra and DAPTACEL were administered concomitantly.
15. References
- 1
- CDC. Guillain-Barré syndrome among recipients of Menactra® meningococcal conjugate vaccine-United States, June 2005-September 2006. MMWR Morb Mortal Wkly Rep 2006;55:1120-1124. Erratum in: MMWR Morb Mortal Wkly Rep 2006;55(43):1177.
- 2
- Harvard Medical School/Harvard Pilgrim Health Care Institute. Risk of Guillain-Barré Syndrome Following Meningococcal Conjugate (MCV4) Vaccination. Final Study Report, Revised March 11, 2010.
- 3
- Mueller JH, et al. A protein-free medium for primary isolation of the gonococcus and meningococcus. Proc Soc Exp Biol Med 1941;48:330-333.
- 4
- Watson RG, et al. The specific hapten of group C (group IIa) meningococcus. I. Preparation and immunological behavior. J Immunol 1958;81:331-336.
- 5
- Mueller JH, et al. Production of diphtheria toxin of high potency (100 Lf) on a reproducible medium. J Immunol 1941;40:21-32.
- 6
- Mäkelä PH, et al. Evolution of conjugate vaccines. Expert Rev Vaccines 2002;1:399-410.
- 7
- Goldschneider I, et al. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 1969;129:1307-1326.
- 8
- Maslanka SE, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin and Diag Lab Immunol 1997;4:156-167.
16. How is Menactra supplied
17. Patient Counseling Information
Vaccine Information Statements are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to immunization to the patient, parent, or guardian. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
Inform the patients, parents or guardians about:
- Potential benefits and risks of immunization with Menactra.
- Potential for adverse reactions that have been temporally associated with administration of Menactra or other vaccines containing similar components.
- Reporting any adverse reactions to their healthcare provider.
- The Sanofi Pasteur Inc. Pregnancy Registry, as appropriate [see Pregnancy (8.1)].
| MENACTRA
neisseria meningitidis group a capsular polysaccharide diphtheria toxoid conjugate antigen, neisseria meningitidis group c capsular polysaccharide diphtheria toxoid conjugate antigen, neisseria meningitidis group y capsular polysaccharide diphtheria toxoid conjugate antigen, and neisseria meningitidis group w-135 capsular polysaccharide diphtheria toxoid conjugate antigen injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sanofi Pasteur Inc. (086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi Pasteur Inc. | 086723285 | MANUFACTURE(49281-589) | |