Drug Detail:Methergine (oral and injection) (Methylergonovine (oral and injection) [ meth-il-er-gon-o-veen ])
Drug Class: Uterotonic agents
Methergine Description
Methergine is available in tablets for oral ingestion containing 0.2 mg methylergonovine maleate.
Tablets
Active ingredient: Methylergonovine maleate, USP, 0.2 mg.
Inactive ingredients: acacia, corn starch, gelatin, lactose monohydrate, methylparaben, microcrystalline cellulose, povidone, propylparaben, stearic acid, and tartaric acid.
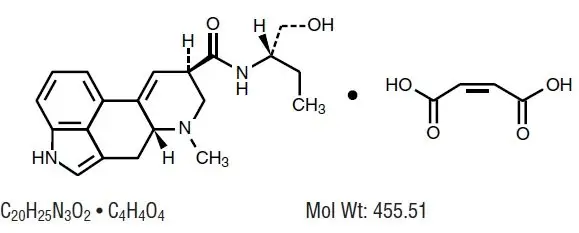
Chemically, methylergonovine maleate is designated as ergoline-8-carboxamide, 9, 10-didehydro- N-[1-(hydroxymethyl) propyl]-6-methyl-, [8β( S)]-, (Z)-2-butenedioate (1:1) (salt).
Its structural formula is:
Methergine - Clinical Pharmacology
Pharmacokinetic studies following an I.V. injection have shown that methylergonovine is rapidly distributed from plasma to peripheral tissues within 2-3 minutes or less. The bioavailability after oral administration was reported to be about 60% with no accumulation after repeated doses. During delivery, with intramuscular injection, bioavailability increased to 78%. Ergot alkaloids are mostly eliminated by hepatic metabolism and excretion, and the decrease in bioavailability following oral administration is probably a result of first-pass metabolism in the liver.
Bioavailability studies conducted in fasting healthy female volunteers have shown that oral absorption of a 0.2 mg methylergonovine tablet was fairly rapid with a mean peak plasma concentration of 3243 ± 1308 pg/mL observed at 1.12 ± 0.82 hours. For a 0.2 mg intramuscular injection, a mean peak plasma concentration of 5918 ± 1952 pg/mL was observed at 0.41 ± 0.21 hours. The extent of absorption of the tablet, based upon methylergonovine plasma concentrations, was found to be equivalent to that of the I.M. solution given orally, and the extent of oral absorption of the I.M. solution was proportional to the dose following administration of 0.1, 0.2, and 0.4 mg. When given intramuscularly, the extent of absorption of Methergine solution was about 25% greater than the tablet. The volume of distribution (Vd ss/F) of methylergonovine was calculated to be 56.1 ± 17.0 liters, and the plasma clearance (CLp/F) was calculated to be 14.4 ± 4.5 liters per hour. The plasma level decline was biphasic with a mean elimination half-life of 3.39 hours (range 1.5 to 12.7 hours). A delayed gastrointestinal absorption (T max about 3 hours) of Methergine tablet might be observed in postpartum women during continuous treatment with this oxytocic agent.
Warnings
Medication errors
Methergine has been administered instead of vitamin K and Hepatitis B vaccine, medications which are routinely administered to the newborn. Due to the potential for accidental neonatal exposure, Methergine injection should be stored separately from medications intended for neonatal administration.
Precautions
Drug Interactions
CYP 3A4 inhibitors (e.g., Macrolide Antibiotics and Protease Inhibitors)
There have been rare reports of serious adverse events in connection with the coadministration of certain ergot alkaloid drugs (e.g., dihydroergotamine and ergotamine) and potent CYP 3A4 inhibitors, resulting in vasospasm leading to cerebral ischemia and/or ischemia of the extremities. Although there have been no reports of such interactions with methylergonovine alone, potent CYP 3A4 inhibitors should not be coadministered with methylergonovine. Examples of some of the more potent CYP 3A4 inhibitors include macrolide antibiotics (e.g., erythromycin, troleandomycin, clarithromycin), HIV protease or reverse transcriptase inhibitors (e.g., ritonavir, indinavir, nelfinavir, delavirdine) or azole antifungals (e.g., ketoconazole, itraconazole, voriconazole). Less potent CYP 3A4 inhibitors should be administered with caution. Less potent inhibitors include saquinavir, nefazodone, fluconazole, grapefruit juice, fluoxetine, fluvoxamine, zileuton, and clotrimazole. These lists are not exhaustive, and the prescriber should consider the effects on CYP 3A4 of other agents being considered for concomitant use with methylergonovine.
CYP3A4 inducers
Drugs (e.g. nevirapine, rifampicin) that are strong inducers of CYP3A4 are likely to decrease the pharmacological action of Methergine.
Beta-blockers
Caution should be exercised when Methergine is used concurrently with beta-blockers. Concomitant administration with beta-blockers may enhance the vasoconstrictive action of ergot alkaloids.
Anesthetics
Anesthetics like halothan and methoxyfluran may reduce the oxytocic potency of Methergine.
Glyceryl trinitrate and other antianginal drugs
Methylergonovine maleate produces vasoconstriction and can be expected to reduce the effect of glyceryl trinitrate and other antianginal drugs.
No pharmacokinetic interactions involving other cytochrome P450 isoenzymes are known.
Caution should be exercised when Methergine (methylergonovine maleate) is used concurrently with other vasoconstrictors, ergot alkaloids, or prostaglandins.
Adverse Reactions/Side Effects
There have been rare isolated reports of anaphylaxis, without a proven causal relationship to the drug product.
Postmarketing Experience
The following adverse drug reactions have been derived from post-marketing experience with Methergine via spontaneous case reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency which is therefore categorized as not known.
Nervous system disorders
Cerebrovascular accident, paraesthesia
Cardiac disorders
Ventricular fibrillation, ventricular tachycardia, angina pectoris, atrioventricular block
Overdosage
Because reports of overdosage with Methergine (methylergonovine maleate) are infrequent, the lethal dose in humans has not been established. The oral LD 50 (in mg/kg) for the mouse is 187, the rat 93, and the rabbit 4.5 2. Several cases of accidental Methergine injection in newborn infants have been reported, and in such cases 0.2 mg represents an overdose of great magnitude. However, recovery occurred in all but one case following a period of respiratory depression, hypothermia, hypertonicity with jerking movements, and convulsions.
Also, several children 1-3 years of age have accidentally ingested up to 10 tablets (2 mg) with no apparent ill effects. A postpartum patient took 4 tablets at one time in error and reported paresthesias and clamminess as her only symptoms.
Treatment of acute overdosage is symptomatic and includes the usual procedures of:
- removal of offending drug by inducing emesis, gastric lavage, catharsis, and supportive diuresis.
- maintenance of adequate pulmonary ventilation, especially if convulsions or coma develop.
- correction of hypotension with pressor drugs as needed.
- control of convulsions with standard anticonvulsant agents.
- control of peripheral vasospasm with warmth to the extremities if needed.
Methergine Dosage and Administration
How is Methergine supplied
Bottles of 7………………………………NDC 27437-050-19
Bottles of 12…………………………….NDC 27437-050-57
Bottles of 28…………………………….NDC 27437-050-56
Bottles of 100………………………...…NDC 27437-050-01
STORE AND DISPENSE
Tablets: Store below 25°C (77°F); in tight, light-resistant container.
Methergine is a registered trademark of Novartis AG
Manufactured by:
Novel Laboratories, Inc.
Somerset, NJ 08873
Manufactured for:
Lupin Pharma
Baltimore, MD 21202
PI1400003901
Iss. 01/2016
| METHERGINE
methylergonovine maleate tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Lupin Pharmaceuticals, Inc. (089153071) |
| Registrant - Novel Laboratories, Inc. (793518643) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novel Laboratories, Inc. | 793518643 | analysis(27437-050) , manufacture(27437-050) | |








