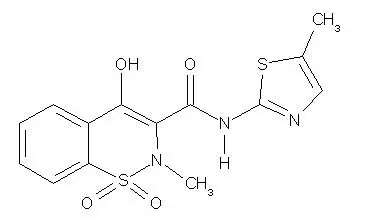
Drug Detail:Mobic (Meloxicam [ mel-oks-i-kam ])
Drug Class: Nonsteroidal anti-inflammatory drugs
Highlights of Prescribing Information
MOBIC® (meloxicam) oral suspension
Initial U.S. Approval: 2000
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
See full prescribing information for complete boxed warning.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use (5.1)
- MOBIC is contraindicated in the setting of coronary artery bypass graft (CABG) surgery (4, 5.1)
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events (5.2)
Recent Major Changes
Indications and Usage for Mobic
MOBIC is a non-steroidal anti-inflammatory drug indicated for:
- Osteoarthritis (OA) (1.1)
- Rheumatoid Arthritis (RA) (1.2)
- Juvenile Rheumatoid Arthritis (JRA) in patients 2 years of age or older (1.3)
Mobic Dosage and Administration
Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals (2.1)
- OA (2.2) and RA (2.3):
- Starting dose: 7.5 mg once daily
- Dose may be increased to 15 mg once daily
- JRA (2.4):
- 0.125 mg/kg once daily up to a maximum of 7.5 mg. JRA dosing using the oral suspension should be individualized based on the weight of the child (2.4)
- MOBIC Suspension is not interchangeable with approved formulations of oral meloxicam even if the total milligram strength is the same (2.6)
Dosage Forms and Strengths
- MOBIC (meloxicam) Oral Suspension: 7.5 mg/5 mL (3)
Contraindications
- Known hypersensitivity to meloxicam or any components of the drug product (4)
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs (4)
- In the setting of CABG surgery (4)
Warnings and Precautions
- Hepatotoxicity: Inform patients of warning signs and symptoms of hepatotoxicity. Discontinue if abnormal liver tests persist or worsen or if clinical signs and symptoms of liver disease develop (5.3)
- Hypertension: Patients taking some antihypertensive medications may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure (5.4, 7)
- Heart Failure and Edema: Avoid use of MOBIC in patients with severe heart failure unless benefits are expected to outweigh risk of worsening heart failure (5.5)
- Renal Toxicity: Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of MOBIC in patients with advanced renal disease unless benefits are expected to outweigh risk of worsening renal function (5.6)
- Anaphylactic Reactions: Seek emergency help if an anaphylactic reaction occurs (5.7)
- Exacerbation of Asthma Related to Aspirin Sensitivity: MOBIC is contraindicated in patients with aspirin-sensitive asthma. Monitor patients with preexisting asthma (without aspirin sensitivity) (5.8)
- Serious Skin Reactions: Discontinue MOBIC at first appearance of skin rash or other signs of hypersensitivity (5.9)
- Premature Closure of Fetal Ductus Arteriosus: Avoid use in pregnant women starting at 30 weeks gestation (5.10, 8.1)
- Hematologic Toxicity: Monitor hemoglobin or hematocrit in patients with any signs or symptoms of anemia (5.11, 7)
Adverse Reactions/Side Effects
- Most common (≥5% and greater than placebo) adverse events in adults are diarrhea, upper respiratory tract infections, dyspepsia, and influenza-like symptoms (6.1)
- Adverse events observed in pediatric studies were similar in nature to the adult clinical trial experience (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or (800) 459-9906 TTY or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Drugs that Interfere with Hemostasis (e.g., warfarin, aspirin, SSRIs/SNRIs): Monitor patients for bleeding who are concomitantly taking MOBIC with drugs that interfere with hemostasis. Concomitant use of MOBIC and analgesic doses of aspirin is not generally recommended (7)
- ACE Inhibitors, Angiotensin Receptor Blockers (ARBs) or Beta-Blockers: Concomitant use with MOBIC may diminish the antihypertensive effect of these drugs. Monitor blood pressure (7)
- ACE Inhibitors and ARBs: Concomitant use with MOBIC in elderly, volume-depleted, or those with renal impairment may result in deterioration of renal function. In such high risk patients, monitor for signs of worsening renal function (7)
- Diuretics: NSAIDs can reduce natriuretic effect of furosemide and thiazide diuretics. Monitor patients to assure diuretic efficacy including antihypertensive effects (7)
Use In Specific Populations
- Pregnancy: Use of NSAIDs during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs in pregnant women starting at 30 weeks gestation (5.10, 8.1)
- Infertility: NSAIDs are associated with reversible infertility. Consider withdrawal of MOBIC in women who have difficulties conceiving (8.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2016
Full Prescribing Information
WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use [see Warnings and Precautions (5.1)].
- MOBIC is contraindicated in the setting of coronary artery bypass graft (CABG) surgery [see Contraindications (4) and Warnings and Precautions (5.1)].
Gastrointestinal Bleeding, Ulceration, and Perforation
- NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events [see Warnings and Precautions (5.2)].
Mobic Dosage and Administration
2.4 Juvenile Rheumatoid Arthritis (JRA) Pauciarticular and Polyarticular Course
| 0.125 mg/kg | ||
| Weight | Dose (1.5 mg/mL) | Delivered dose |
| 12 kg (26 lb) | 1.0 mL | 1.5 mg |
| 24 kg (54 lb) | 2.0 mL | 3.0 mg |
| 36 kg (80 lb) | 3.0 mL | 4.5 mg |
| 48 kg (106 lb) | 4.0 mL | 6.0 mg |
| ≥60 kg (132 lb) | 5.0 mL | 7.5 mg |
Dosage Forms and Strengths
MOBIC (meloxicam) Oral Suspension:
- yellowish green tinged viscous suspension containing 7.5 mg meloxicam per 5 mL.
Contraindications
MOBIC is contraindicated in the following patients:
- Known hypersensitivity (e.g., anaphylactic reactions and serious skin reactions) to meloxicam or any components of the drug product [see Warnings and Precautions (5.7, 5.9)]
- History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, sometimes fatal, anaphylactic reactions to NSAIDs have been reported in such patients [see Warnings and Precautions (5.7, 5.8)]
- In the setting of coronary artery bypass graft (CABG) surgery [see Warnings and Precautions (5.1)]
Warnings and Precautions
5.2 Gastrointestinal Bleeding, Ulceration, and Perforation
Strategies to Minimize the GI Risks in NSAID-treated patients:
- Use the lowest effective dosage for the shortest possible duration.
- Avoid administration of more than one NSAID at a time.
- Avoid use in patients at higher risk unless benefits are expected to outweigh the increased risk of bleeding. For such patients, as well as those with active GI bleeding, consider alternate therapies other than NSAIDs.
- Remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy.
- If a serious GI adverse event is suspected, promptly initiate evaluation and treatment, and discontinue MOBIC until a serious GI adverse event is ruled out.
- In the setting of concomitant use of low-dose aspirin for cardiac prophylaxis, monitor patients more closely for evidence of GI bleeding [see Drug Interactions (7)].
Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Thrombotic Events [see Boxed Warning and Warnings and Precautions (5.1)]
- GI Bleeding, Ulceration, and Perforation [see Boxed Warning and Warnings and Precautions (5.2)]
- Hepatotoxicity [see Warnings and Precautions (5.3)]
- Hypertension [see Warnings and Precautions (5.4)]
- Heart Failure and Edema [see Warnings and Precautions (5.5)]
- Renal Toxicity and Hyperkalemia [see Warnings and Precautions (5.6)]
- Anaphylactic Reactions [see Warnings and Precautions (5.7)]
- Serious Skin Reactions [see Warnings and Precautions (5.9)]
- Hematologic Toxicity [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
| 1 WHO preferred terms edema,
edema dependent, edema peripheral, and edema legs combined 2 WHO preferred terms rash, rash erythematous, and rash maculo-papular combined | |||||
| Placebo | MOBIC 7.5 mg daily | MOBIC 15 mg daily | Diclofenac 100 mg daily | ||
| No. of Patients | 157 | 154 | 156 | 153 | |
| Gastrointestinal | 17.2 | 20.1 | 17.3 | 28.1 | |
| Abdominal pain | 2.5 | 1.9 | 2.6 | 1.3 | |
| Diarrhea | 3.8 | 7.8 | 3.2 | 9.2 | |
| Dyspepsia | 4.5 | 4.5 | 4.5 | 6.5 | |
| Flatulence | 4.5 | 3.2 | 3.2 | 3.9 | |
| Nausea | 3.2 | 3.9 | 3.8 | 7.2 | |
| Body as a Whole | |||||
| Accident household | 1.9 | 4.5 | 3.2 | 2.6 | |
| Edema1 | 2.5 | 1.9 | 4.5 | 3.3 | |
| Fall | 0.6 | 2.6 | 0.0 | 1.3 | |
| Influenza-like symptoms | 5.1 | 4.5 | 5.8 | 2.6 | |
| Central and Peripheral Nervous System |
|||||
| Dizziness | 3.2 | 2.6 | 3.8 | 2.0 | |
| Headache | 10.2 | 7.8 | 8.3 | 5.9 | |
| Respiratory | |||||
| Pharyngitis | 1.3 | 0.6 | 3.2 | 1.3 | |
| Upper respiratory tract infection | 1.9 | 3.2 | 1.9 | 3.3 | |
| Skin | |||||
| Rash2 | 2.5 | 2.6 | 0.6 | 2.0 | |
| 1 MedDRA high level term
(preferred terms): dyspeptic signs and symptoms (dyspepsia, dyspepsia
aggravated, eructation, gastrointestinal irritation), upper respiratory
tract infections-pathogen unspecified (laryngitis NOS, pharyngitis
NOS, sinusitis NOS), joint related signs and symptoms (arthralgia,
arthralgia aggravated, joint crepitation, joint effusion, joint swelling) 2 MedDRA preferred term: nausea, abdominal pain NOS, influenza-like illness, headaches NOS, and rash NOS | ||||
| Placebo | MOBIC 7.5 mg daily | MOBIC 15 mg daily | ||
| No. of Patients | 469 | 481 | 477 | |
| Gastrointestinal Disorders | 14.1 | 18.9 | 16.8 | |
| Abdominal pain NOS2 | 0.6 | 2.9 | 2.3 | |
| Dyspeptic signs and symptoms1 | 3.8 | 5.8 | 4.0 | |
| Nausea2 | 2.6 | 3.3 | 3.8 | |
| General Disorders and Administration Site Conditions | ||||
| Influenza-like illness2 | 2.1 | 2.9 | 2.3 | |
| Infection and Infestations
Upper respiratory tract infections-pathogen class unspecified1 | 4.1 | 7.0 | 6.5 | |
| Musculoskeletal and Connective
Tissue Disorders
Joint related signs and symptoms1 | 1.9 | 1.5 | 2.3 | |
| Nervous System Disorders | ||||
| Headaches NOS2 | 6.4 | 6.4 | 5.5 | |
| Skin and Subcutaneous Tissue Disorders
Rash NOS2 | 1.7 | 1.0 | 2.1 | |
| 1 WHO preferred terms edema,
edema dependent, edema peripheral, and edema legs combined 2 WHO preferred terms rash, rash erythematous, and rash maculo-papular combined |
||||
| 4 to 6 Weeks Controlled Trials | 6 Month Controlled Trials | |||
| MOBIC 7.5 mg daily | MOBIC 15 mg daily | MOBIC 7.5 mg daily | MOBIC 15 mg daily |
|
| No. of Patients | 8955 | 256 | 169 | 306 |
| Gastrointestinal | 11.8 | 18.0 | 26.6 | 24.2 |
| Abdominal pain | 2.7 | 2.3 | 4.7 | 2.9 |
| Constipation | 0.8 | 1.2 | 1.8 | 2.6 |
| Diarrhea | 1.9 | 2.7 | 5.9 | 2.6 |
| Dyspepsia | 3.8 | 7.4 | 8.9 | 9.5 |
| Flatulence | 0.5 | 0.4 | 3.0 | 2.6 |
| Nausea | 2.4 | 4.7 | 4.7 | 7.2 |
| Vomiting | 0.6 | 0.8 | 1.8 | 2.6 |
| Body as a Whole | ||||
| Accident household | 0.0 | 0.0 | 0.6 | 2.9 |
| Edema1 | 0.6 | 2.0 | 2.4 | 1.6 |
| Pain | 0.9 | 2.0 | 3.6 | 5.2 |
| Central and Peripheral Nervous System | ||||
| Dizziness | 1.1 | 1.6 | 2.4 | 2.6 |
| Headache | 2.4 | 2.7 | 3.6 | 2.6 |
| Hematologic | ||||
| Anemia | 0.1 | 0.0 | 4.1 | 2.9 |
| Musculoskeletal | ||||
| Arthralgia | 0.5 | 0.0 | 5.3 | 1.3 |
| Back pain | 0.5 | 0.4 | 3.0 | 0.7 |
| Psychiatric | ||||
| Insomnia | 0.4 | 0.0 | 3.6 | 1.6 |
| Respiratory | ||||
| Coughing | 0.2 | 0.8 | 2.4 | 1.0 |
| Upper respiratory tract infection | 0.2 | 0.0 | 8.3 | 7.5 |
| Skin | ||||
| Pruritus | 0.4 | 1.2 | 2.4 | 0.0 |
| Rash2 | 0.3 | 1.2 | 3.0 | 1.3 |
| Urinary | ||||
| Micturition frequency | 0.1 | 0.4 | 2.4 | 1.3 |
| Urinary tract infection | 0.3 | 0.4 | 4.7 | 6.9 |
Pauciarticular and Polyarticular Course Juvenile Rheumatoid Arthritis (JRA)
| Body as a Whole | allergic reaction, face edema, fatigue, fever, hot flushes, malaise, syncope, weight decrease, weight increase |
| Cardiovascular | angina pectoris, cardiac failure, hypertension, hypotension, myocardial infarction, vasculitis |
| Central and Peripheral Nervous System | convulsions, paresthesia, tremor, vertigo |
| Gastrointestinal | colitis, dry mouth, duodenal ulcer, eructation, esophagitis, gastric ulcer, gastritis, gastroesophageal reflux, gastrointestinal hemorrhage, hematemesis, hemorrhagic duodenal ulcer, hemorrhagic gastric ulcer, intestinal perforation, melena, pancreatitis, perforated duodenal ulcer, perforated gastric ulcer, stomatitis ulcerative |
| Heart Rate and Rhythm | arrhythmia, palpitation, tachycardia |
| Hematologic | leukopenia, purpura, thrombocytopenia |
| Liver and Biliary System | ALT increased, AST increased, bilirubinemia, GGT increased, hepatitis |
| Metabolic and Nutritional | dehydration |
| Psychiatric | abnormal dreaming, anxiety, appetite increased, confusion, depression, nervousness, somnolence |
| Respiratory | asthma, bronchospasm, dyspnea |
| Skin and Appendages | alopecia, angioedema, bullous eruption, photosensitivity reaction, pruritus, sweating increased, urticaria |
| Special Senses | abnormal vision, conjunctivitis, taste perversion, tinnitus |
| Urinary System | albuminuria, BUN increased, creatinine increased, hematuria, renal failure |
Drug Interactions
| Drugs that Interfere with Hemostasis | |
| Clinical Impact:
|
|
| Intervention:
| Monitor patients with concomitant use of MOBIC with anticoagulants (e.g., warfarin), antiplatelet agents (e.g., aspirin), selective serotonin reuptake inhibitors (SSRIs), and serotonin norepinephrine reuptake inhibitors (SNRIs) for signs of bleeding [see Warnings and Precautions (5.11)]. |
| Aspirin | |
| Clinical Impact:
| Controlled clinical studies showed that the concomitant use of NSAIDs and analgesic doses of aspirin does not produce any greater therapeutic effect than the use of NSAIDs alone. In a clinical study, the concomitant use of an NSAID and aspirin was associated with a significantly increased incidence of GI adverse reactions as compared to use of the NSAID alone [see Warnings and Precautions (5.2)]. |
| Intervention:
| Concomitant
use of MOBIC and low dose aspirin or analgesic doses of aspirin is
not generally recommended because of the increased risk of bleeding
[see Warnings and Precautions (5.11)]. MOBIC is not a substitute for low dose aspirin for cardiovascular protection. |
| ACE Inhibitors, Angiotensin Receptor Blockers, or Beta-Blockers | |
| Clinical Impact:
|
|
| Intervention:
|
|
| Diuretics | |
| Clinical Impact:
| Clinical studies, as well as post-marketing observations, showed that NSAIDs reduced the natriuretic effect of loop diuretics (e.g., furosemide) and thiazide diuretics in some patients. This effect has been attributed to the NSAID inhibition of renal prostaglandin synthesis. However, studies with furosemide agents and meloxicam have not demonstrated a reduction in natriuretic effect. Furosemide single and multiple dose pharmacodynamics and pharmacokinetics are not affected by multiple doses of meloxicam. |
| Intervention:
| During concomitant use of MOBIC with diuretics, observe patients for signs of worsening renal function, in addition to assuring diuretic efficacy including antihypertensive effects [see Warnings and Precautions (5.6)]. |
| Lithium | |
| Clinical Impact:
| NSAIDs have produced elevations in plasma lithium levels and reductions in renal lithium clearance. The mean minimum lithium concentration increased 15%, and the renal clearance decreased by approximately 20%. This effect has been attributed to NSAID inhibition of renal prostaglandin synthesis [see Clinical Pharmacology (12.3)]. |
| Intervention:
| During concomitant use of MOBIC and lithium, monitor patients for signs of lithium toxicity. |
| Methotrexate | |
| Clinical Impact:
| Concomitant use of NSAIDs and methotrexate may increase the risk for methotrexate toxicity (e.g., neutropenia, thrombocytopenia, renal dysfunction). |
| Intervention:
| During concomitant use of MOBIC and methotrexate, monitor patients for methotrexate toxicity. |
| Cyclosporine | |
| Clinical Impact:
| Concomitant use of MOBIC and cyclosporine may increase cyclosporine's nephrotoxicity. |
| Intervention:
| During concomitant use of MOBIC and cyclosporine, monitor patients for signs of worsening renal function. |
| NSAIDs and Salicylates | |
| Clinical Impact:
| Concomitant use of meloxicam with other NSAIDs or salicylates (e.g., diflunisal, salsalate) increases the risk of GI toxicity, with little or no increase in efficacy [see Warnings and Precautions (5.2)]. |
| Intervention:
| The concomitant use of meloxicam with other NSAIDs or salicylates is not recommended. |
| Pemetrexed | |
| Clinical Impact:
| Concomitant use of MOBIC and pemetrexed may increase the risk of pemetrexed-associated myelosuppression, renal, and GI toxicity (see the pemetrexed prescribing information). |
| Intervention:
| During concomitant use of MOBIC and pemetrexed, in patients
with renal impairment whose creatinine clearance ranges from 45 to
79 mL/min, monitor for myelosuppression, renal and GI toxicity. Patients taking meloxicam should interrupt dosing for at least five days before, the day of, and two days following pemetrexed administration. In patients with creatinine clearance below 45 mL/min, the concomitant administration of meloxicam with pemetrexed is not recommended. |
| Kayexalate® (sodium polystyrene sulfonate) | |
| Clinical Impact:
| Cases of intestinal necrosis (possibly fatal) have been described in patients who received concomitant sorbitol and Kayexalate® (sodium polystyrene sulfonate). Due to the presence of sorbitol in MOBIC Oral Suspension, use with Kayexalate® is not recommended. |
| Intervention:
| The concomitant use of MOBIC Oral Suspension with Kayexalate® is not recommended. |
Use In Specific Populations
8.2 Lactation
Meloxicam was present in the milk of lactating rats at concentrations higher than those in plasma.
Mobic - Clinical Pharmacology
12.3 Pharmacokinetics
| 1The parameter values in
the table are from various studies 2 not under high fat conditions 3 MOBIC tablets 4 Vz/f =Dose/(AUC•Kel) |
||||||
| Steady State | Single Dose | |||||
| Pharmacokinetic Parameters (% CV) | Healthy
male adults (Fed)2 | Elderly
males (Fed)2 | Elderly females (Fed)2 | Renal failure (Fasted) | Hepatic insufficiency (Fasted) | |
| 7.5 mg3 tablets | 15 mg capsules | 15 mg capsules | 15 mg capsules | 15 mg capsules | ||
| N | 18 | 5 | 8 | 12 | 12 | |
| Cmax | [µg/mL] | 1.05 (20) | 2.3 (59) | 3.2 (24) | 0.59 (36) | 0.84 (29) |
| tmax | [h] | 4.9 (8) | 5 (12) | 6 (27) | 4 (65) | 10 (87) |
| t1/2 | [h] | 20.1 (29) | 21 (34) | 24 (34) | 18 (46) | 16 (29) |
| CL/f | [mL/min] | 8.8 (29) | 9.9 (76) | 5.1 (22) | 19 (43) | 11 (44) |
| Vz/f4 | [L] | 14.7 (32) | 15 (42) | 10 (30) | 26 (44) | 14 (29) |
The pharmacokinetics of MOBIC in pediatric patients under 2 years of age have not been investigated.
Clinical Studies
How is Mobic supplied
MOBIC (meloxicam) oral suspension 7.5 mg/5 mL: NDC 0597-0034-01; Bottles of 100 mL
Patient Counseling Information
Cardiovascular Thrombotic Events
Gastrointestinal Bleeding, Ulceration, and Perforation
Avoid Concomitant Use of NSAIDs
Use of NSAIDs and Low-Dose Aspirin
Kayexalate is a registered trademark of Sanofi-Aventis

Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA
Licensed from: Boehringer Ingelheim International GmbH
Copyright 2016 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
| Medication Guide for Nonsteroidal Anti-inflammatory Drugs (NSAIDs) | |
| What is the
most important information I should know about medicines called Nonsteroidal
Anti-inflammatory Drugs (NSAIDs)?
NSAIDs can cause serious side effects, including:
|
|
| What are NSAIDs?
NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain. |
|
| Who should
not take NSAIDs?
Do not take NSAIDs:
|
|
Before taking
NSAIDs, tell your healthcare provider about all of your medical conditions,
including if you:
|
|
| What are the
possible side effects of NSAIDs?
NSAIDs can cause serious side effects, including: See “What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?”
These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
Other information
about NSAIDs:
|
|
| General information
about the safe and effective use of NSAIDs
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which it was not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals. |
|
| Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT 06877 USA Licensed from: Boehringer Ingelheim International GmbH Boehringer Ingelheim Pharmaceuticals, Inc. either owns or uses the trademark Mobic® under license. The other trademarks referenced are owned by third parties not affiliated with Boehringer Ingelheim Pharmaceuticals, Inc. Copyright 2016 Boehringer Ingelheim International GmbH ALL RIGHTS RESERVED IT7614AI072016 |
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: May 2016
| MOBIC
meloxicam suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| West-Ward Columbus Inc. | 058839929 | ANALYSIS(0597-0034) , MANUFACTURE(0597-0034) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Promeco S.A de C.V. | 812579472 | PACK(0597-0034) , LABEL(0597-0034) , ANALYSIS(0597-0034) , MANUFACTURE(0597-0034) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | ANALYSIS(0597-0034) , MANUFACTURE(0597-0034) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bidachem S.p.A. | 429232812 | ANALYSIS(0597-0034) , API MANUFACTURE(0597-0034) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 340700520 | ANALYSIS(0597-0034) , API MANUFACTURE(0597-0034) | |