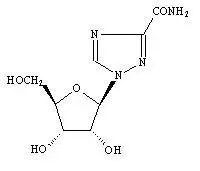
Drug Detail:Moderiba (Ribavirin (oral) [ rye-ba-vye-rin ])
Drug Class: Purine nucleosides
Highlights of Prescribing Information
Moderiba™ (ribavirin, USP) Tablets for oral use
Initial U.S. Approval: 2002
WARNING: RISK OF SERIOUS DISORDERS AND RIBAVIRIN-ASSOCIATED EFFECTS
See full prescribing information for complete boxed warning.
- Ribavirin monotherapy, including Moderiba, is not effective for the treatment of chronic hepatitis C virus infection (Boxed Warning).
- The hemolytic anemia associated with ribavirin therapy may result in worsening of cardiac disease and lead to fatal and nonfatal myocardial infarctions. Patients with a history of significant or unstable cardiac disease should not be treated with Moderiba (2.3, 5.2, 6.1).
-
Significant teratogenic and embryocidal effects have been demonstrated in all animal species exposed to ribavirin. Therefore, Moderiba is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Extreme care must be taken to avoid pregnancy during therapy and for 6 months after completion of treatment in both female patients and in female partners of male patients who are taking Moderiba therapy (4, 5.1, 8.1).
Recent Major Changes
Indications and Usage for Moderiba
Moderiba is a nucleoside analogue indicated for the treatment of chronic hepatitis C (CHC) virus infection in combination with peginterferon alfa-2a in patients 5 years of age and older with compensated liver disease not previously treated with interferon alpha, and in adult CHC patients coinfected with HIV (1)
Moderiba Dosage and Administration
- CHC: Moderiba is administered according to body weight and genotype (2.1)
- CHC with HIV coinfection: 800 mg by mouth daily for a total of 48 weeks, regardless of genotype (2.2)
- Dose reduction or discontinuation is recommended in patients experiencing certain adverse reactions or renal impairment (2.3, 2.4)
Dosage Forms and Strengths
- Moderiba (ribavirin, USP) tablets 200 mg (3)
- Moderiba (ribavirin, USP) tablets 400 mg (3)
- Moderiba (ribavirin, USP) tablets 600 mg (3)
Contraindications
- Pregnant women and men whose female partners are pregnant (4, 5.1, 8.1)
- Hemoglobinopathies (4)
- Coadministration with didanosine (4, 7.1)
Moderiba in combination with peginterferon alfa-2a is contraindicated in patients with:
- Autoimmune hepatitis (4)
- Hepatic decompensation in cirrhotic patients (4, 5.3)
Warnings and Precautions
- Birth defects and fetal death with ribavirin: Do not use in pregnancy and for 6 months after treatment. Patients must have a negative pregnancy test prior to therapy, use at least 2 forms of contraception and undergo monthly pregnancy tests (4, 5.1, 8.1)
Peginterferon alfa-2a/Moderiba: Patients exhibiting the following conditions should be closely monitored and may require dose reduction or discontinuation of therapy:
- Hemolytic anemia may occur with a significant initial drop in hemoglobin. This may result in worsening cardiac disease leading to fatal or nonfatal myocardial infarctions (5.2, 6.1)
- Risk of hepatic failure and death: Monitor hepatic function during treatment and discontinue treatment for hepatic decompensation (5.3)
- Severe hypersensitivity reactions including urticaria, angioedema, bronchoconstriction, and anaphylaxis, and serious skin reactions such as Stevens-Johnson Syndrome (5.4)
- Pulmonary disorders, including pulmonary function impairment and pneumonitis, including fatal cases of pneumonia (5.5)
- Severe depression and suicidal ideation, autoimmune and infectious disorders, suppression of bone marrow function, pancreatitis, and diabetes (5)
- Bone marrow suppression with azathioprine coadministration (5.6)
- Growth impairment with combination therapy in pediatric patients (5.8)
Adverse Reactions/Side Effects
The most common adverse reactions (frequency greater than 40%) in adults receiving combination therapy are fatigue/asthenia, pyrexia, myalgia, and headache. (6.1)
The most common adverse reactions in pediatric subjects were similar to those seen in adults. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Nucleoside analogues: Closely monitor for toxicities. Discontinue nucleoside reverse transcriptase inhibitors or reduce dose or discontinue interferon, ribavirin or both with worsening toxicities (7.1)
- Azathioprine: Concomitant use of azathioprine with ribavirin has been reported to induce severe pancytopenia and may increase the risk of azathioprine-related myelotoxicity (7.3)
Use In Specific Populations
- Ribavirin Pregnancy Registry (8.1)
- Pediatrics: Safety and efficacy in pediatric patients less than 5 years old have not been established (8.4)
- Renal Impairment: Dose should be reduced in patients with creatinine clearance less than or equal to 50 mL/min (8.7)
- Organ Transplant: Safety and efficacy have not been studied (8.10)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2017
Full Prescribing Information
1. Indications and Usage for Moderiba
- This indication is based on clinical trials of combination therapy in patients with CHC and compensated liver disease, some of whom had histological evidence of cirrhosis (Child-Pugh class A), and in adult patients with clinically stable HIV disease and CD4 count greater than 100 cells/mm3.
- This indication is based on achieving undetectable HCV-RNA after treatment for 24 or 48 weeks, based on HCV genotype, and maintaining a Sustained Virologic Response (SVR) 24 weeks after the last dose.
- Safety and efficacy data are not available for treatment longer than 48 weeks.
- The safety and efficacy of ribavirin and peginterferon alfa-2a therapy have not been established in liver or other organ transplant recipients, patients with decompensated liver disease, or previous non-responders to interferon therapy.
- The safety and efficacy of ribavirin therapy for the treatment of adenovirus, RSV, parainfluenza or influenza infections have not been established. Moderiba should not be used for these indications. Ribavirin for inhalation has a separate package insert, which should be consulted if ribavirin inhalation therapy is being considered.
2. Moderiba Dosage and Administration
2.1 Chronic Hepatitis C Monoinfection
| Hepatitis C Virus (HCV) Genotype | Peginterferon alfa-2a Dose* (once weekly) | Moderiba Dose (daily) | Duration |
| Genotypes 1, 4 | 180 mcg | <75 kg = 1000 mg ≥75 kg = 1200 mg | 48 weeks 48 weeks |
| Genotypes 2, 3 | 180 mcg | 800 mg | 24 weeks |
| Genotypes 2 and 3 showed no increased response to treatment beyond 24 weeks (see Table 10). Data on genotypes 5 and 6 are insufficient for dosing recommendations. *See Peginterferon alfa-2a Package Insert for further details on peginterferon alfa-2a dosing and administration, including dose modification in patients with renal impairment. |
|||
| Body Weight in kilograms (kg) | Moderiba Daily Dose* | Moderiba Number of Tablets |
| 23 – 33 | 400 mg/day | 1 x 200 mg tablet A.M. 1 x 200 mg tablet P.M. |
| 34 – 46 | 600 mg/day | 1 x 200 mg tablet A.M. 2 x 200 mg tablets P.M.** |
| 47 – 59 | 800 mg/day | 2 x 200 mg tablets A.M.** 2 x 200 mg tablets P.M.** |
| 60 – 74 | 1000 mg/day | 2 x 200 mg tablets A.M.** 3 x 200 mg tablets P.M.*** |
| ≥75 | 1200 mg/day | 3 x 200 mg tablets A.M.*** 3 x 200 mg tablets P.M.*** |
| *approximately 15 mg/kg/day **or 1 x 400 mg tablet ***or 1 x 600 mg tablet |
||
2.3 Dose Modifications
| Body weight in kilograms (kg) | Laboratory Values | |
| Hemoglobin <10 g/dL in patients with no cardiac disease, or Decrease in hemoglobin of ≥2 g/dL during any 4 week period in patients with history of stable cardiac disease | Hemoglobin <8.5 g/dL in patients with no cardiac disease, or Hemoglobin <12 g/dL despite 4 weeks at reduced dose in patients with history of stable cardiac disease |
|
| Adult Patients older than 18 years of age | ||
| Any weight | 1 x 200 mg tablet A.M. 2 x 200 mg tablets or 1 x 400 mg tablet P.M. | Discontinue Moderiba |
| Pediatric Patients 5 to 18 years of age | ||
| 23 – 33 kg | 1 x 200 mg tablet A.M. | Discontinue Moderiba |
| 34 – 46 kg | 1 x 200 mg tablet A.M. 1 x 200 mg tablet P.M. |
|
| 47 – 59 kg | 1 x 200 mg tablet A.M. 1 x 200 mg tablet P.M. |
|
| 60 – 74 kg | 1 x 200 mg tablet A.M. 2 x 200 mg tablets P.M. or 1 x 400 mg tablet P.M. |
|
| ≥75 kg | 1 x 200 mg tablet A.M. 2 x 200 mg tablets P.M. or 1 x 400 mg tablet P.M. |
|
2.4 Renal Impairment
| Creatinine Clearance | Peginterferon alfa-2a Dose (once weekly) | Moderiba Dose (daily) |
| 30 to 50 mL/min | 180 mcg | Alternating doses, 200 mg and 400 mg every other day |
| Less than 30 mL/min | 135 mcg | 200 mg daily |
| Hemodialysis | 135 mcg | 200 mg daily |
No data are available for pediatric subjects with renal impairment.
3. Dosage Forms and Strengths
Moderiba (ribavirin, USP) is available as tablets for oral administration.
4. Contraindications
Moderiba (ribavirin, USP) is contraindicated in:
- Women who are pregnant. Moderiba may cause fetal harm when administered to a pregnant woman. Moderiba is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus [see Warnings and Precautions (5.1), Use in Specific Populations (8.1), and Patient Counseling Information (17)].
- Men whose female partners are pregnant.
- Patients with hemoglobinopathies (e.g., thalassemia major or sickle-cell anemia).
- In combination with didanosine. Reports of fatal hepatic failure, as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis have been reported in clinical trials [see Drug Interactions (7.1)].
Moderiba and peginterferon alfa-2a combination therapy is contraindicated in patients with:
- Autoimmune hepatitis.
- Hepatic decompensation (Child-Pugh score greater than 6; class B and C) in cirrhotic CHC monoinfected patients before treatment [see Warnings and Precautions (5.3)].
- Hepatic decompensation (Child-Pugh score greater than or equal to 6) in cirrhotic CHC patients coinfected with HIV before treatment [see Warnings and Precautions (5.3)].
5. Warnings and Precautions
5.9 Laboratory Tests
- Platelet count greater than or equal to 90,000 cells/mm3 (as low as 75,000 cells/mm3 in HCV patients with cirrhosis or 70,000 cells/mm3 in patients with CHC and HIV)
- Absolute neutrophil count (ANC) greater than or equal to 1500 cells/mm3
- TSH and T4 within normal limits or adequately controlled thyroid function
- CD4+ cell count greater than or equal to 200 cells/mm3 or CD4+ cell count greater than or equal to 100 cells/mm3 but less than 200 cells/mm3 and HIV-1 RNA less than 5,000 copies/mL in patients coinfected with HIV
- Hemoglobin greater than or equal to 12 g/dL for women and greater than or equal to 13 g/dL for men in CHC monoinfected patients
- Hemoglobin greater than or equal to 11 g/dL for women and greater than or equal to 12 g/dL for men in patients with CHC and HIV
6. Adverse Reactions/Side Effects
6.1 Clinical Studies Experience
| Body System | CHC Combination Therapy Study NV15801 |
|
| peginterferon alfa-2a 180 mcg + 1000 mg or 1200 mg ribavirin tablets 48 weeks | interferon alfa-2b + 1000 mg or 1200 mg ribavirin capsules 48 weeks |
|
| N=451 | N=443 | |
| % | % | |
| Application Site Disorders | ||
| Injection site reaction | 23 | 16 |
| Endocrine Disorders | ||
| Hypothyroidism | 4 | 5 |
| Flu-like Symptoms and Signs | ||
| Fatigue/Asthenia | 65 | 68 |
| Pyrexia | 41 | 55 |
| Rigors | 25 | 37 |
| Pain | 10 | 9 |
| Gastrointestinal | ||
| Nausea/Vomiting | 25 | 29 |
| Diarrhea | 11 | 10 |
| Abdominal pain | 8 | 9 |
| Dry mouth | 4 | 7 |
| Dyspepsia | 6 | 5 |
| Hematologic* | ||
| Lymphopenia | 14 | 12 |
| Anemia | 11 | 11 |
| Neutropenia | 27 | 8 |
| Thrombocytopenia | 5 | <1 |
| Metabolic and Nutritional | ||
| Anorexia | 24 | 26 |
| Weight decrease | 10 | 10 |
| Musculoskeletal, Connective Tissue and Bone | ||
| Myalgia | 40 | 49 |
| Arthralgia | 22 | 23 |
| Back pain | 5 | 5 |
| Neurological | ||
| Headache | 43 | 49 |
| Dizziness (excluding vertigo) | 14 | 14 |
| Memory impairment | 6 | 5 |
| Psychiatric | ||
| Irritability/Anxiety/Nervousness | 33 | 38 |
| Insomnia | 30 | 37 |
| Depression | 20 | 28 |
| Concentration impairment | 10 | 13 |
| Mood alteration | 5 | 6 |
| Resistance Mechanism Disorders | ||
| Overall | 12 | 10 |
| Respiratory, Thoracic and Mediastinal | ||
| Dyspnea | 13 | 14 |
| Cough | 10 | 7 |
| Dyspnea exertional | 4 | 7 |
| Skin and Subcutaneous Tissue | ||
| Alopecia | 28 | 33 |
| Pruritus | 19 | 18 |
| Dermatitis | 16 | 13 |
| Dry skin | 10 | 13 |
| Rash | 8 | 5 |
| Sweating increased | 6 | 5 |
| Eczema | 5 | 4 |
| Visual Disorders | ||
| Vision blurred | 5 | 2 |
| *Severe hematologic abnormalities (lymphocyte less than 500 cells/mm3; hemoglobin less than 10 g/dL; neutrophil less than 750 cells/mm3; platelet less than 50,000 cells/mm3). | ||
| Study NV17424 | ||
| System Organ Class | peginterferon alfa-2a 180 mcg/1.73 m2 x BSA + ribavirin tablets 15 mg/kg (N=55) | peginterferon alfa-2a 180 mcg/1.73 m2 x BSA + Placebo** (N=59) |
| % | % | |
| General disorders and administration site conditions | ||
| Influenza like illness | 91 | 81 |
| Injection site reaction | 44 | 42 |
| Fatigue | 25 | 20 |
| Irritability | 24 | 14 |
| Gastrointestinal disorders | ||
| Gastrointestinal disorder | 49 | 44 |
| Nervous system disorders | ||
| Headache | 51 | 39 |
| Skin and subcutaneous tissue disorders | ||
| Rash | 15 | 10 |
| Pruritus | 11 | 12 |
| Musculoskeletal, connective tissue and bone disorders | ||
| Musculoskeletal pain | 35 | 29 |
| Psychiatric disorders | ||
| Insomnia | 9 | 12 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 11 | 14 |
| *Displayed adverse drug reactions include all grades of reported adverse clinical events considered possibly, probably, or definitely related to study drug. **Subjects in the peginterferon alfa-2a plus placebo arm who did not achieve undetectable viral load at week 24 switched to combination treatment thereafter. Therefore, only the first 24 weeks are presented for the comparison of combination therapy with monotherapy. |
||
Growth Inhibition in Pediatric Subjects [see Warnings and Precautions (5.8)].
Common Adverse Reactions in CHC with HIV Coinfection (Adults)
| Laboratory Parameter | Peginterferon alfa-2a + Ribavirin 1000/1200 mg 48 wks | Interferon alfa-2b + Ribavirin 1000/1200 mg 48 wks |
| (N=887) | (N=443) | |
| Neutrophils (cells/mm3) | ||
| 1,000 <1,500 | 34% | 38% |
| 500 <1,000 | 49% | 21% |
| <500 | 5% | 1% |
| Platelets (cells/mm3) | ||
| 50,000 – <75,000 | 11% | 4% |
| 20,000 – <50,000 | 5% | <1% |
| <20,000 | 0 | 0 |
| Hemoglobin (g/dL) | ||
| 8.5 – 9.9 | 11% | 11% |
| <8.5 | 2% | <1% |
| Laboratory Parameter | Peginterferon alfa-2a 180 mcg/1.73 m2 x BSA + Ribavirin tablets 15 mg/kg (N=55) | Peginterferon alfa-2a 180 mcg/1.73 m2 x BSA + Placebo* (N=59) |
| Neutrophils (cells/mm3) | ||
| 1,000 - <1,500 | 31% | 39% |
| 750 - <1,000 | 27% | 17% |
| 500 - <750 | 25% | 15% |
| <500 | 7% | 5% |
| Platelets (cells/mm3) | ||
| 75,000 - <100,000 | 4% | 2% |
| 50,000 - <75,000 | 0% | 2% |
| <50,000 | 0% | 0% |
| Hemoglobin (g/dL) | ||
| 8.5 – <10 | 7% | 3% |
| <8.5 | 0% | 0% |
| *Subjects in the peginterferon alfa-2a plus placebo arm who did not achieve undetectable viral load at week 24 switched to combination treatment thereafter. Therefore, only the first 24 weeks are presented for the comparison of combination therapy with monotherapy. | ||
7. Drug Interactions
7.2 Drugs Metabolized by Cytochrome P450
In vitro studies indicate that ribavirin does not inhibit CYP 2C9, CYP 2C19, CYP 2D6 or CYP 3A4.
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy: Category X [see Contraindications (4)].
8.9 Gender
Ribavirin pharmacokinetics, when corrected for weight, are similar in male and female patients.
12. Moderiba - Clinical Pharmacology
13. Nonclinical Toxicology
14. Clinical Studies
14.1 Chronic Hepatitis C Patients
| Interferon alfa-2b + Ribavirin 1000 mg or 1200 mg | Peginterferon alfa-2a + placebo | Peginterferon alfa-2a + Ribavirin Tablets 1000 mg or 1200 mg |
|
|---|---|---|---|
| All patients | 197/444 (44%) | 65/224 (29%) | 241/453 (53%) |
| Genotype 1 | 103/285 (36%) | 29/145 (20%) | 132/298 (44%) |
| Genotypes 2–6 | 94/159 (59%) | 36/79 (46%) | 109/155 (70%) |
| Difference in overall treatment response (Peginterferon alfa-2a/ribavirin – Interferon alfa-2b/ribavirin) was 9% (95% CI 2.3, 15.3). | |||
Sustained Virologic Response (SVR) and HCV Genotype
The numbers of patients with genotype 5 and 6 were too few to allow for meaningful assessment.
| 24 Weeks Treatment | 48 Weeks Treatment | |||
| Peginterferon alfa-2a + Ribavirin 800 mg (N=207) | Peginterferon alfa-2a + Ribavirin 1000 mg or 1200 mg* (N=280) | Peginterferon alfa-2a + Ribavirin 800 mg (N=361) | Peginterferon alfa-2a + Ribavirin 1000 mg or 1200 mg* (N=436) |
|
| Genotype 1 | 29/101 (29%) | 48/118 (41%) | 99/250 (40%) | 138/271 (51%) |
| Genotypes 2, 3 | 79/96 (82%) | 116/144 (81%) | 75/99 (76%) | 117/153 (76%) |
| Genotype 4 | 0/5 (0%) | 7/12 (58%) | 5/8 (63%) | 9/11 (82%) |
| *1000 mg for body weight less than 75 kg; 1200 mg for body weight greater than or equal to 75 kg. | ||||
| Peginterferon alfa-2a 180 mcg/1.73 m2 x BSA + Ribavirin 15 mg/kg* (N=55) | Peginterferon alfa-2a 180 mcg/1.73 m2 x BSA + Placebo* (N=59) |
|
| All HCV genotypes** | 29 (53%) | 12 (20%) |
| HCV genotype 1 | 21/45 (47%) | 8/47 (17%) |
| HCV non-genotype 1*** | 8/10 (80%) | 4/12 (33%) |
| *Results indicate undetectable HCV-RNA defined as HCV RNA less than 50 IU/mL at 24 weeks post-treatment using the AMPLICOR HCV test v2 **Scheduled treatment duration was 48 weeks regardless of the genotype ***Includes HCV genotypes 2, 3 and others |
||
14.3 Chronic Hepatitis C/HIV Coinfected Patients
| Interferon alfa-2a + Ribavirin 800 mg (N=289) | peginterferon alfa-2a + Placebo (N=289) | peginterferon alfa-2a + Ribavirin 800 mg (N=290) |
|
| All patients | 33 (11%) | 58 (20%) | 116 (40%) |
| Genotype 1 | 12/171 (7%) | 24/175 (14%) | 51/176 (29%) |
| Genotypes 2, 3 | 18/89 (20%) | 32/90 (36%) | 59/95 (62%) |
16. How is Moderiba supplied
Moderiba (ribavirin, USP) is available as tablets for oral administration.
200 mg Bottles of 168 NDC 0074-3197-16
Moderiba™ is also available in blister packs as follows:
Moderiba™ 600 Dose Pack Carton
Moderiba™ 800 Dose Pack Carton
Moderiba™ 1000 Dose Pack Carton
17. Patient Counseling Information
- See FDA-approved patient labeling (Medication Guide)
Moderiba™ (Mah-duh-RYE-bah)
(ribavirin, USP)
Tablets
Also read the Medication Guide for PEGASYS1 (peginterferon alfa-2a).
What is the most important information I should know about Moderiba?
- You should not take Moderiba alone to treat chronic hepatitis C infection. Moderiba should be used with peginterferon alfa-2a to treat chronic hepatitis C infection.
- Moderiba may cause you to have a blood problem (hemolytic anemia) that can worsen any heart problems you have, and cause you to have a heart attack or die. Tell your healthcare provider if you have ever had any heart problems. Moderiba may not be right for you. If you have chest pain while you take Moderiba, get emergency medical attention right away.
- Moderiba may cause birth defects or death of your unborn baby. If you are pregnant or your sexual partner is pregnant, do not take Moderiba. You or your sexual partner should not become pregnant while you take Moderiba and for 6 months after treatment is over. You must use two forms of birth control when you take Moderiba and for the 6 months after treatment.
- Females must have a pregnancy test before starting Moderiba, every month while treated with Moderiba, and every month for the 6 months after treatment with Moderiba.
- If you or your female sexual partner becomes pregnant while taking Moderiba or within 6 months after you stop taking Moderiba, tell your healthcare provider right away. You or your healthcare provider should contact the Ribavirin Pregnancy Registry by calling 1-800-593-2214. The Ribavirin Pregnancy Registry collects information about what happens to mothers and their babies if the mother takes Moderiba while she is pregnant.
See “What is the most important information I should know about Moderiba?”
- have certain types of hepatitis caused by your immune system attacking your liver (autoimmune hepatitis)
- have certain blood disorders, such as thalassemia major or sickle-cell anemia (hemoglobinopathies)
- take didanosine (Videx®2 or Videx EC®2)
What should I tell my healthcare provider before taking Moderiba?
Before you take Moderiba, tell your healthcare provider if you have or have had:
- treatment for hepatitis C that did not work for you
- serious allergic reactions to Moderiba or to any of the ingredients in Moderiba. See the end of this Medication Guide for a list of ingredients.
- breathing problems. Moderiba may cause or worsen your breathing problems you already have.
- vision problems. Moderiba may cause eye problems or worsen eye problems you already have. You should have an eye exam before you start treatment with Moderiba.
- certain blood disorders such as anemia
- high blood pressure, heart problems or have had a heart attack. Your healthcare provider should test your blood and heart before you start treatment with Moderiba.
- thyroid problems
- diabetes. Moderiba and peginterferon alfa-2a combination therapy may make your diabetes worse or harder to treat.
- liver problems other than hepatitis C virus infection
- human immunodeficiency virus (HIV) or other immunity problems
- mental health problems, including depression or thoughts of suicide
- kidney problems
- an organ transplant
- drug addiction or abuse
- infection with hepatitis B virus
- any other medical condition
- are breast feeding. It is not known if Moderiba passes into your breast milk. You and your healthcare provider should decide if you will take Moderiba or breast-feed.
- Take Moderiba exactly as your healthcare provider tells you. Your healthcare provider will tell you how much Moderiba to take and when to take it. For children 5 years of age and older your healthcare provider will prescribe the dose of Moderiba based on weight.
- Take Moderiba with food.
- If you miss a dose of Moderiba, take the missed dose as soon as possible during the same day. Do not double the next dose. If you have questions about what to do, call your healthcare provider.
- If you take too much Moderiba, call your healthcare provider or local Poison Control Center right away, or go to the nearest hospital emergency room right away.
- Your healthcare provider should do blood tests before you start treatment with Moderiba, at weeks 2 and 4 of treatment, and then as needed to see how well you are tolerating treatment and to check for side effects. Your healthcare provider may change your dose of Moderiba based on blood test results or side effects you may have.
- If you have heart problems, your healthcare provider should check your heart by doing an electrocardiogram before you start treatment with Moderiba, and if needed during treatment.
What should I avoid while taking Moderiba?
- Moderiba can make you feel tired, dizzy, or confused. You should not drive or operate machinery if you have any of these symptoms.
- Do not drink alcohol, including beer, wine, and liquor. This may make your liver disease worse.
What are the possible side effects of Moderiba?
Moderiba may cause serious side effects including:
See “What is the most important information I should know about Moderiba?”
- Swelling and irritation of your pancreas (pancreatitis). You may have stomach pain, nausea, vomiting or diarrhea.
- Severe allergic reactions. Symptoms may include hives, wheezing, trouble breathing, chest pain, swelling of your mouth, tongue, or lips, or severe rash.
- Serious breathing problems. Difficulty breathing may be a sign of a serious lung infection (pneumonia) that can lead to death.
- Serious eye problems that may lead to vision loss or blindness.
- Liver problems. Some people may get worsening of liver function. Tell your healthcare provider right away if you have any of these symptoms: stomach bloating, confusion, brown urine, and yellow eyes.
- Severe depression
- Suicidal thoughts and attempts
- Effect on growth in children. Children can experience a delay in weight gain and height increase while being treated with peginterferon alfa-2a and Moderiba. Catch-up in growth happens after treatment stops, but some children may not reach the height that they were expected to have before treatment. Talk to your healthcare provider if you are concerned about your child’s growth during treatment with peginterferon alfa-2a and Moderiba.
Common side effects of Moderiba taken with peginterferon alfa-2a include:
- flu-like symptoms-feeling tired, headache, shaking along with high temperature (fever), and muscle or joint aches
- mood changes, feeling irritable, anxiety, and difficulty sleeping
- loss of appetite, nausea, vomiting, and diarrhea
- hair loss
- itching
Tell your healthcare provider about any side effect that bothers you or that does not go away.
You may also report side effects to AbbVie Inc. at 1-800-633-9110.
- Store Moderiba tablets between 59°F and 86°F (15°C and 30°C).
- Keep the bottle tightly closed.
Keep Moderiba and all medicines out of the reach of children.
General information about the safe and effective use of Moderiba
What are the ingredients in Moderiba?
This Medication Guide has been approved by the U.S. Food and Drug Administration.
1 PEGASYS is a trademark of Hoffmann-La Roche, Inc.
2 Videx and Videx EC is a registered trademark of Bristol-Myers Squibb Company
3 Imuran is a registered trademark of Prometheus Laboratories, Inc.
4 Azasan is a registered trademark of Salix Pharmaceuticals, Inc.
C139.00023 – Revised December, 2017
Copyright © 2015 by Kadmon Pharmaceuticals, LLC. All rights reserved.
AVOID PREGNANCY WHILE TAKING THIS MEDICATION. READ THE MEDICATION GUIDE FOR IMPORTANT INFORMATION.






AVOID PREGNANCY WHILE TAKING THIS MEDICATION. READ THE MEDICATION GUIDE FOR IMPORTANT INFORMATION.
Contains 14 ribavirin tablets (600 mg each)
AM Dose: 600 mg / PM Dose: 600 mg
Patient instructions for each daily dose:
AM - In the morning, push one ribavirin tablet, 600 mg through the cardboard backing.
PM - In the evening, push one ribavirin tablet, 600 mg through the cardboard backing.
Please read the enclosed Medication Guide
56 Tablets in 4 Unit Dose Packs
14 ribavirin tablets (600 mg each)
AVOID PREGNANCY WHILE TAKING THIS MEDICATION. READ THE MEDICATION GUIDE FOR IMPORTANT INFORMATION.
| MODERIBA
ribavirin kit |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| MODERIBA
ribavirin kit |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| MODERIBA
ribavirin tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| MODERIBA
ribavirin tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| MODERIBA
ribavirin tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - AbbVie Inc. (078458370) |