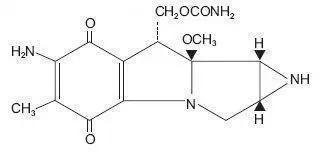
Drug Detail:Mutamycin (Mitomycin [ mye-toe-mye-sin ])
Drug Class: Antibiotics / antineoplastics
Rx only
Warnings
Patients being treated with mitomycin must be observed carefully and frequently during and after therapy.
The use of mitomycin results in a high incidence of bone marrow suppression, particularly thrombocytopenia and leukopenia. Therefore, the following studies should be obtained repeatedly during therapy and for at least eight weeks following therapy: platelet count, white blood cell count, differential, and hemoglobin. The occurrence of a platelet count below 100,000/mm 3 or a WBC below 4,000/mm 3 or a progressive decline in either is an indication to withhold further therapy until blood counts have recovered above these levels.
Patients should be advised of the potential toxicity of this drug, particularly bone marrow suppression. Deaths have been reported due to septicemia as a result of leukopenia due to the drug.
Patients receiving mitomycin should be observed for evidence of renal toxicity. Mitomycin should not be given to patients with a serum creatinine greater than 1.7 mg percent.
Precautions
Acute shortness of breath and severe bronchospasm have been reported following the administration of vinca alkaloids in patients who had previously or simultaneously received mitomycin. The onset of this acute respiratory distress occurred within minutes to hours after the vinca alkaloid injection. The total number of doses for each drug has varied considerably. Bronchodilators, steroids and/or oxygen have produced symptomatic relief.
A few cases of adult respiratory distress syndrome have been reported in patients receiving mitomycin in combination with other chemotherapy and maintained at FlO 2 concentrations greater than 50% perioperatively. Therefore, caution should be exercised using only enough oxygen to provide adequate arterial saturation since oxygen itself is toxic to the lungs. Careful attention should be paid to fluid balance and overhydration should be avoided.
Bladder fibrosis/contraction has been reported with intravesical administration (not an approved route of administration), which in rare cases has required cystectomy.
Geriatric Use
Insufficient data from clinical studies of mitomycin are available for patients 65 years of age and older to determine whether they respond differently than younger patients. Postmarketing surveillance suggests that elderly patients may be more susceptible than younger patients to injection site reactions (see ADVERSE REACTIONS: Integument and Mucous Membrane Toxicity) and hypersensitivity reactions. In general, caution should be exercised when prescribing to elderly patients, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Adverse Reactions/Side Effects
Integument and Mucous Membrane Toxicity
This has occurred in approximately 4% of patients treated with mitomycin. Cellulitis at the injection site has been reported and is occasionally severe. Stomatitis and alopecia also occur frequently. Rashes are rarely reported. The most important dermatological problem with this drug, however, is the necrosis and consequent sloughing of tissue which results if the drug is extravasated during injection. Extravasation may occur with or without an accompanying stinging or burning sensation and even if there is adequate blood return when the injection needle is aspirated. There have been reports of delayed erythema and/or ulceration occurring either at or distant from the injection site, weeks to months after mitomycin, even when no obvious evidence of extravasation was observed during administration. Skin grafting has been required in some of the cases. Elderly patients may be more susceptible than younger patients to injection site reactions (see PRECAUTIONS: Geriatric Use).
Manufactured for :
Accord BioPharma Inc.
1009 Slater Road, Suite 210-B,
Durham, NC 27703. USA
Manufactured by :
Intas Pharmaceuticals Limited,
Ahmedabad – 380 009, India.
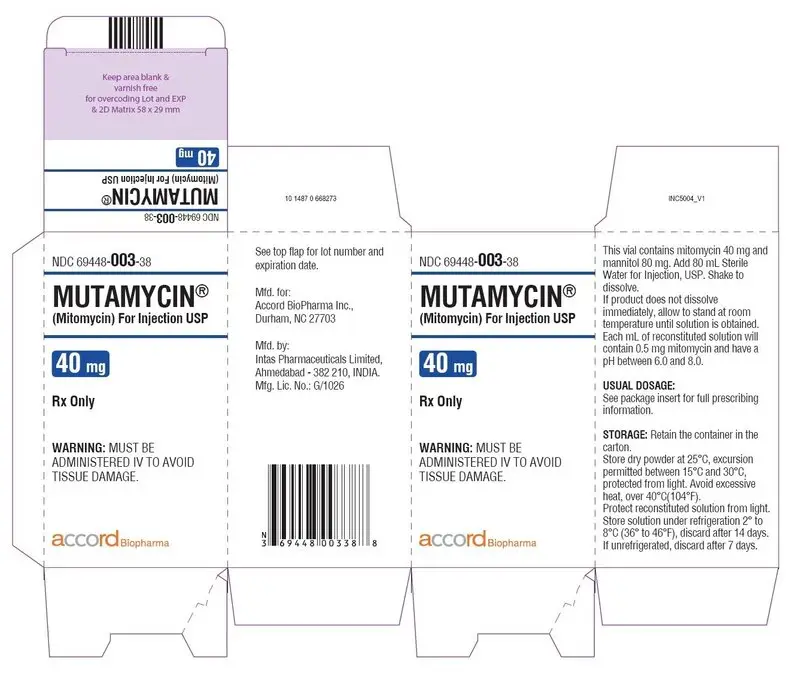
Carton Label-MUTAMYCIN ® (Mitomycin) for injection USP 40 mg/vial
NDC 69448- 003-38
MUTAMYCIN
®
(Mitomycin) For Injection USP
40 mg
Rx only
WARNING:
MUST BE ADMINISTERED IV TO AVOID
TISSUE DAMAGE
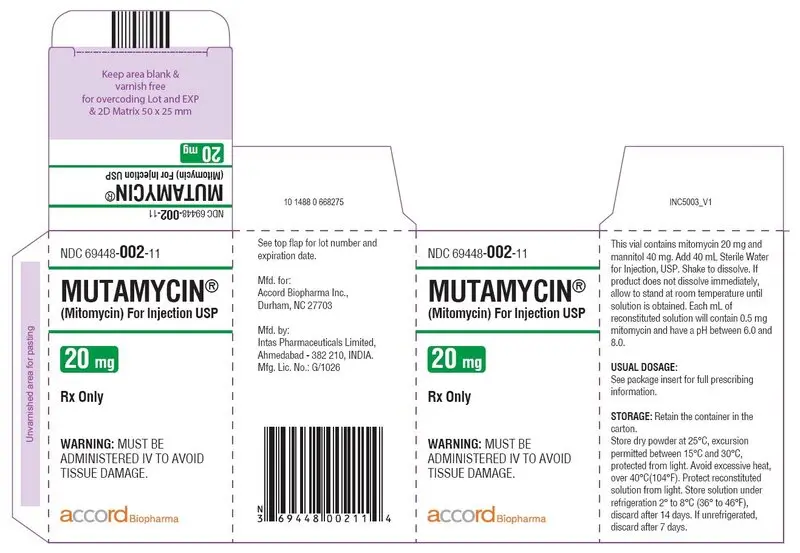
Carton Label-MUTAMYCIN
® (Mitomycin) for injection USP 20 mg/vial
NDC 69448-
002-11
MUTAMYCIN
®
(Mitomycin) For Injection USP
20 mg
Rx only
WARNING:
MUST BE ADMINISTERED IV TO AVOID
TISSUE DAMAGE
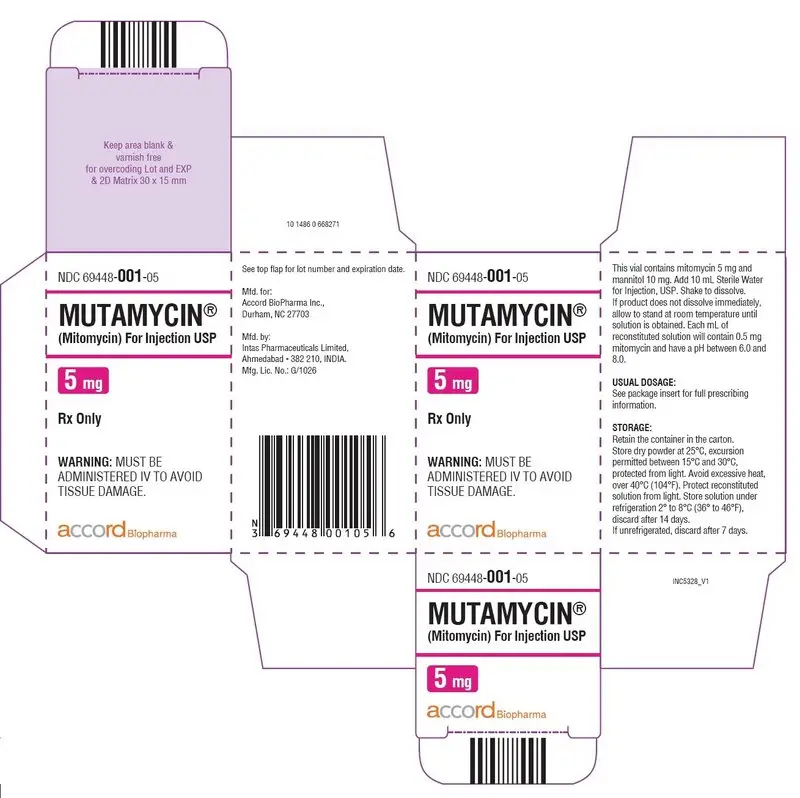
Carton Label-MUTAMYCIN ® (Mitomycin) for injection USP 5 mg/vial
NDC 69448- 001-05
MUTAMYCIN
®
(Mitomycin) For Injection USP
5 mg
Rx only
WARNING:
MUST BE ADMINISTERED IV TO AVOID
TISSUE DAMAGE
| MUTAMYCIN
mitomycin injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| MUTAMYCIN
mitomycin injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| MUTAMYCIN
mitomycin injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Accord BioPharma Inc. (079636487) |
| Registrant - Accord Healthcare Inc. (604222237) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Intas Pharmaceuticals Limited | 725927649 | manufacture(69448-003, 69448-002, 69448-001) , analysis(69448-003, 69448-002, 69448-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Intas Pharmaceuticals Limited | 915837971 | manufacture(69448-003, 69448-002, 69448-001) , analysis(69448-003, 69448-002, 69448-001) | |