Drug Detail:Nayzilam nasal spray (Midazolam (nasal) [ my-daz-oh-lam ])
Drug Class: Benzodiazepines
Highlights of Prescribing Information
NAYZILAM® (midazolam) nasal spray, CIV
Initial U.S. Approval: 1985
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
See full prescribing information for complete boxed warning.
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death (5.1, 7.2)
- The use of benzodiazepines, including NAYZILAM, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Before prescribing NAYZILAM and throughout treatment, assess each patient's risk for abuse, misuse, and addiction (5.2).
- Although NAYZILAM is indicated only for intermittent use (1, 2), if used more frequently than recommended, abrupt discontinuation or rapid dosage reduction of NAYZILAM may precipitate acute withdrawal reactions, which can be life-threatening. For patients using NAYZILAM more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue NAYZILAM (5.3).
Recent Major Changes
| Warnings and Precautions (5.9) | 1/2023 |
Indications and Usage for Nayzilam
NAYZILAM is a benzodiazepine indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy 12 years of age and older. (1)
Nayzilam Dosage and Administration
Administer NAYZILAM by the nasal route only. (2.2)
Initial Dose: Administer one spray (5 mg dose) into one nostril. (2.2)
Second Dose: One additional spray (5 mg dose) into the opposite nostril may be administered after 10 minutes if the patient has not responded to the initial dose. (2.2)
Maximum Dosage and Treatment Frequency: Do not use more than 2 doses of NAYZILAM to treat a seizure cluster. It is recommended that NAYZILAM be used to treat no more than one episode every three days and treat no more than five episodes per month. (2.2)
Dosage Forms and Strengths
Single-dose nasal spray unit containing 5 mg midazolam per 0.1 mL solution. (3)
Contraindications
- Patients with hypersensitivity to midazolam (4)
- Patients with acute narrow-angle glaucoma (4)
Warnings and Precautions
- CNS Depression From Concomitant Use With Other CNS Depressants or Moderate or Strong CYP3A4 Inhibitors: May cause an increased CNS-depressant effect when used with alcohol or other CNS depressants. Concomitant use with moderate or strong CYP3A4 inhibitors may result in prolonged sedation because of a decrease in plasma clearance of midazolam. (5.5, 7.3)
- Suicidal Behavior and Ideation: Antiepileptic drugs increase the risk of suicidal ideation and behavior. (5.6)
- Impaired Cognitive Function: Midazolam is associated with a high incidence of partial or complete impairment of recall for the next several hours. (5.7)
- Glaucoma: NAYZILAM can increase intraocular pressure in patients with glaucoma. Patients with open-angle glaucoma may need to have their ophthalmologic status evaluated following treatment with NAYZILAM. (5.8)
- Neonatal Sedation and Withdrawal Syndrome: NAYZILAM use during pregnancy can result in neonatal sedation and/or neonatal withdrawal. (5.9, 8.1)
Adverse Reactions/Side Effects
The most common adverse reactions (≥5% in any NAYZILAM treatment group) were somnolence, headache, nasal discomfort, throat irritation, and rhinorrhea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact UCB, Inc. at 1-844-599-2273 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 Inhibitors: Avoid co-administration of NAYZILAM with moderate or strong CYP3A4 inhibitors. NAYZILAM should be used with caution when co-administered with mild CYP3A4 inhibitors. (7.1)
- Opioids: Risk of respiratory depression is increased. (7.2)
- Other CNS Depressants: May increase the risks of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect. (7.3)
Use In Specific Populations
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Lactation: Midazolam is excreted in human milk. Caution should be exercised when NAYZILAM is administered to a nursing woman. (8.2)
- Renal Impairment: Patients with renal impairment may have longer elimination half-lives for midazolam and its metabolites which may result in prolonged exposure. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2023
Full Prescribing Information
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation [see Warnings and Precautions (5.1) and Drug Interactions (7.2)].
- The use of benzodiazepines, including NAYZILAM, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing NAYZILAM and throughout treatment, assess each patient's risk for abuse, misuse, and addiction [see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Although NAYZILAM is indicated only for intermittent use [see Indications and Usage (1) and Dosage and Administration (2)], if used more frequently than recommended abrupt discontinuation or rapid dosage reduction of NAYZILAM may precipitate acute withdrawal reactions, which can be life-threatening. For patients using NAYZILAM more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue NAYZILAM [see Warnings and Precautions (5.3)].
1. Indications and Usage for Nayzilam
NAYZILAM is indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy 12 years of age and older.
2. Nayzilam Dosage and Administration
2.1 Instructions Prior to Dosing
NAYZILAM prescribers should consider the following prior to initiation of treatment:
For patients at increased risk of respiratory depression from benzodiazepines, administration of NAYZILAM under healthcare professional supervision should be considered prior to treatment with NAYZILAM; this administration may be performed in the absence of a seizure episode [see Warnings and Precautions (5.4)].
Prior to treatment, the healthcare professional should instruct the individual administering NAYZILAM on how to identify seizure clusters and use the product appropriately [see Patient Counseling Information: Administration Information (17)]. Patients and caregivers should be counseled to read carefully the "Instructions for Use" for complete directions on how to properly administer NAYZILAM.
3. Dosage Forms and Strengths
NAYZILAM is supplied as a single-dose nasal spray unit containing 5 mg of midazolam in 0.1 mL solution.
4. Contraindications
NAYZILAM is contraindicated in patients with:
- Known hypersensitivity to midazolam.
- Acute narrow-angle glaucoma [see Warnings and Precautions (5.8)].
5. Warnings and Precautions
5.1 Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including NAYZILAM, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of benzodiazepines and opioids for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe NAYZILAM concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. Advise both patients and caregivers about the risks of respiratory depression and sedation when NAYZILAM is used with opioids [see Drug Interactions (7.2)].
5.2 Abuse, Misuse, and Addiction
The use of benzodiazepines, including NAYZILAM, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death [see Drug Abuse and Dependence (9.2)].
Before prescribing NAYZILAM and throughout treatment, assess each patient's risk for abuse, misuse, and addiction. Use of NAYZILAM, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of NAYZILAM along with monitoring for signs and symptoms of abuse, misuse, and addiction. Do not exceed the recommended dosing frequency; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
5.3 Dependence and Withdrawal Reactions After Use of NAYZILAM More Frequently Than Recommended
For patients using NAYZILAM more frequently than recommended, to reduce the risk of withdrawal reactions, use a gradual taper to discontinue NAYZILAM (a patient-specific plan should be used to taper the dose). Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
5.4 Risks of Cardiorespiratory Adverse Reactions
Serious cardiorespiratory adverse reactions have occurred after administration of midazolam. These have included respiratory depression, airway obstruction, oxygen desaturation, apnea, respiratory arrest and/or cardiac arrest, sometimes resulting in death or permanent neurologic injury. There have also been rare reports of hypotensive episodes requiring treatment during or after diagnostic or surgical manipulations, particularly in patients with hemodynamic instability. Hypotension occurs more frequently in patients premedicated with a narcotic. The danger of hypoventilation, airway obstruction, or apnea is greater in elderly patients and those with chronic disease states or decreased pulmonary reserve [see Use in Specific Populations (8.5)]; patients with chronic obstructive pulmonary disease are highly sensitive to the respiratory depressant effect of midazolam.
Respiratory depression was observed with the administration of NAYZILAM during clinical trials [see Adverse Reactions (6.1)]. Cardiac or respiratory arrest caused by NAYZILAM was not reported during clinical trials.
5.5 Central Nervous System Depression from Concomitant Use with Other Central Nervous System Depressants, or Moderate or Strong CYP3A4 Inhibitors
Drug products containing midazolam, including NAYZILAM, have a central nervous system (CNS) depressant effect.
5.6 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including NAYZILAM, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed. The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
| Indication | Placebo Patients with Events/1000 Patients | Drug Patients with Events per 1000 Patients | Relative Risk: Incidence of Drug Events in Drug Patients /Incidence in Placebo Patients | Risk Difference: Additional Drug Patients with Events per 1000 Patients |
|---|---|---|---|---|
| Epilepsy | 1.0 | 3.4 | 3.5 | 2.4 |
| Psychiatric | 5.7 | 8.5 | 1.5 | 2.9 |
| Other | 1.0 | 1.8 | 1.9 | 0.9 |
| Total | 2.4 | 4.3 | 1.8 | 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing midazolam or any other AED must balance the risk of suicidal thoughts or behaviors with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
5.7 Impaired Cognitive Function
Midazolam, including NAYZILAM, is associated with a high incidence of partial or complete impairment of recall for several hours following an administered dose. Gross tests of recovery from the effects of midazolam cannot be relied upon to predict reaction time under stress. It is recommended that no patient operate hazardous machinery or a motor vehicle until the effects of the drug, such as drowsiness, have subsided, and as their medical condition permits. For pediatric patients, particular care should be taken to ensure safe ambulation.
5.8 Glaucoma
Benzodiazepines, including NAYZILAM, can increase intraocular pressure in patients with glaucoma. Measurements of intraocular pressure in patients without eye disease show a moderate lowering following induction with midazolam. NAYZILAM may be used in patients with open-angle glaucoma only if they are receiving appropriate therapy. Patients with open-angle glaucoma may need to have their ophthalmologic status evaluated following treatment with NAYZILAM. NAYZILAM is contraindicated in patients with narrow-angle glaucoma.
5.9 Neonatal Sedation and Withdrawal Syndrome
Use of NAYZILAM late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate [see Use in Specific Populations (8.1)]. Monitor neonates exposed to NAYZILAM during pregnancy or labor for signs of sedation and monitor neonates exposed to NAYZILAM during pregnancy for signs of withdrawal; manage these neonates accordingly.
5.10 Other Adverse Reactions
When midazolam was used for sedation, reactions such as agitation, involuntary movements (including tonic/clonic movements and muscle tremor), hyperactivity, and combativeness have been reported. These reactions may be caused by inadequate or excessive dosing or improper administration of midazolam; however, consideration should be given to the possibility of cerebral hypoxia or true paradoxical reactions.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
- Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- Abuse, Misuse, and Addiction [see Warnings and Precautions (5.2)]
- Dependence and Withdrawal Reactions After Use of NAYZILAM More Frequently Than Recommended [see Warnings and Precautions (5.3)]
- Risks of Cardiorespiratory Adverse Reactions [see Warnings and Precautions (5.4)]
- CNS Depression from Concomitant Use with Other CNS Depressants or Moderate or Strong CYP3A4 Inhibitors [see Warnings and Precautions (5.5)]
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.6)]
- Impaired Cognitive Function [see Warnings and Precautions (5.7)]
- Glaucoma [see Warnings and Precautions (5.8)]
- Neonatal Sedation and Withdrawal Syndrome [see Warnings and Precautions (5.9)]
- Other Adverse Reactions [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
NAYZILAM was studied for the outpatient treatment of a single seizure cluster in 292 adult and adolescent patients with epilepsy (Study 1) [see Clinical Studies (14)]. The study was conducted in two phases; an open-label Test Dose Phase followed by a double-blind, placebo-controlled, Comparative Phase. The mean age of patients enrolled in the Comparative Phase (N=201) was 33 years, 51% were female, and 95% were White.
Table 2 lists the adverse reactions occurring in 2% or more of the NAYZILAM-treated patients and at a rate greater than the placebo-treated patients in the Comparative Phase of Study 1.
| Body System/Adverse Reaction | Placebo | NAYZILAM† | |||
|---|---|---|---|---|---|
| NAYZILAM 5 mg | Placebo + NAYZILAM 5 mg | NAYZILAM 5 mg + 5 mg | Any NAYZILAM Treatment Group | ||
| N = 26 % | N = 91 % | N = 41 % | N = 43 % | N = 175 % |
|
|
|||||
| Nervous System | |||||
| Somnolence | 4 | 10 | 10 | 9 | 10 |
| Headache | 0 | 7 | 0 | 2 | 4 |
| Dysarthria | 0 | 2 | 2 | 2 | 2 |
| Application Site | |||||
| Nasal Discomfort | 8 | 5 | 7 | 16 | 9 |
| Throat Irritation | 0 | 2 | 2 | 7 | 3 |
| Rhinorrhea | 0 | 3 | 0 | 5 | 3 |
| Product Taste Abnormal | 0 | 4 | 0 | 0 | 2 |
| Eye Disorders | |||||
| Lacrimation Increased | 0 | 1 | 2 | 2 | 2 |
For patients who experienced a decrease in peripheral oxygen saturation in the Test Dose Phase of Study 1, the decreases were generally transitory. Two patients (one with a history of sleep apnea and one with intercurrent seizure) with decreases in peripheral oxygen saturation in the Test Dose Phase required therapeutic supplemental oxygen.
7. Drug Interactions
| 7.1 CYP3A4 Inhibitors | |
| Clinical Impact: | Concomitant use of CYP3A4 inhibitors may result in prolonged sedation because of a decrease in plasma clearance of midazolam. |
| Intervention: | Avoid co-administration of NAYZILAM with moderate or strong CYP3A4 inhibitors. NAYZILAM should be used with caution when co-administered with mild CYP3A4 inhibitors. |
| Examples: | Moderate CYP3A4 inhibitors: erythromycin, diltiazem, verapamil Strong CYP3A4 inhibitors: ketoconazole, itraconazole, clarithromycin |
| 7.2 Opioids | |
| Clinical Impact: | The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. |
| Intervention: | Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required [see Warnings and Precautions (5.1)]. |
| Examples: | Morphine, hydrocodone, oxymorphone, codeine, fentanyl |
| 7.3 Other Central Nervous System (CNS) Depressants | |
| Clinical Impact: | Concomitant use of barbiturates, alcohol, or other CNS depressants may increase the risk of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect. |
| Intervention: | Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required [see Warnings and Precautions (5.5)]. |
| Examples: | Other benzodiazepines and sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, opioids, alcohol. |
8. Use In Specific Populations
8.1 Pregnancy
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Animal Data
When midazolam (0, 0.2, 1, or 4 mg/kg/day) was administered intravenously to pregnant rats during the period of organogenesis, no adverse effects on embryofetal development were observed. The highest dose tested, which was associated with minimal evidence of maternal toxicity, is approximately 4 times the maximum recommended human dose (MRHD) of 10 mg based on body surface area (mg/m2).
When midazolam (0, 0.2, 0.6, and 2 mg/kg/day) was administered intravenously to rabbits during the period of organogenesis, no adverse effects on embryofetal development were reported. The high dose, which was not associated with evidence of maternal toxicity, is approximately 4 times the MRHD on a mg/m2 basis.
When midazolam (0, 0.2, 1, or 4 mg/kg/day) was administered intravenously to female rats during late gestation and throughout lactation, no clear adverse effects were noted in the offspring. The high dose, which was not associated with evidence of maternal toxicity, is approximately 4 times the MRHD on a mg/m2 basis.
In published animal studies, administration of benzodiazepines, including midazolam, or other drugs that enhance GABAergic neurotransmission to neonatal rats has been reported to result in widespread apoptotic neurodegeneration in the developing brain at plasma concentrations relevant for seizure control in humans. The window of vulnerability to these changes in rats (postnatal days 0-14) includes a period of brain development corresponding to that taking place during the third trimester of pregnancy in humans.
8.4 Pediatric Use
Safety and effectiveness of NAYZILAM have been evaluated in the age group 12 to 17 years. Use of NAYZILAM in this age group is supported by evidence from an adequate and well-controlled study of NAYZILAM in adults and adolescents with seizure clusters [see Clinical Studies (14)] and pharmacokinetic and safety data from adult and pediatric patients [see Clinical Pharmacology (12.3)].
Safety and effectiveness in pediatric patients below the age of 12 years have not been established.
8.5 Geriatric Use
Safety and efficacy studies of NAYZILAM did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Geriatric patients have longer elimination half-lives for midazolam and its metabolites, which may result in prolonged drug exposure. Geriatric patients may have altered drug distribution; diminished hepatic and/or renal function; and subjects over 70 years of age may be particularly sensitive [see Clinical Pharmacology (12.3)]. Administration of intramuscular (IM) midazolam to elderly patients has been associated with rare reports of death under circumstances compatible with cardiorespiratory depression [see Warnings and Precautions (5.4)]. In most of these cases, the patients also received other CNS depressants capable of depressing respiration, especially narcotics [see Warnings and Precautions (5.1, 5.5)]. Close monitoring of geriatric patients is recommended.
8.6 Renal Impairment
Based on a population pharmacokinetic analysis of patients administered NAYZILAM, midazolam and 1-OH midazolam pharmacokinetics are expected to be similar in subjects with mild renal impairment when compared to normal subjects. Safety and efficacy studies of NAYZILAM did not include patients with severe renal impairment and there were not enough subjects with moderate renal impairment in clinical studies for population pharmacokinetic analysis. Patients with moderate and severe renal impairment may have slower elimination of midazolam and its metabolites, which may result in prolonged drug exposure [see Clinical Pharmacology (12.3)].
9. Drug Abuse and Dependence
9.2 Abuse
NAYZILAM is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders [see Warnings and Precautions (5.2)].
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
Midazolam was actively self-administered in primate models used to assess the positive reinforcing effects of psychoactive drugs. Midazolam produced physical dependence of a mild to moderate intensity in cynomolgus monkeys after 5 to 10 weeks of administration.
Assessment of the abuse-related subjective effects comparing NAYZILAM to oral midazolam syrup was conducted in adult subjects with a history of benzodiazepine recreational drug use. No statistically significant or clinically-relevant differences in subjective positive effects (i.e., Drug Liking, Overall Drug Liking, Take Drug Again, and High) were observed between NAYZILAM and oral midazolam syrup. However, subjective positive effects on all these measures were significantly greater for NAYZILAM than for placebo confirming that NAYZILAM has abuse potential. Somnolence occurred at a similar rate in both midazolam groups, but euphoric mood occurred at a greater rate in NAYZILAM (4 to 16%) compared to the oral midazolam syrup (4 to 8.5%).
9.3 Dependence
9.4 Chronic Use
NAYZILAM is not recommended for chronic, daily use as an anticonvulsant because of the potential for development of tolerance to midazolam. In clinical trials, patients were treated with NAYZILAM no more frequently than every 3 days.
Chronic daily use of benzodiazepines may increase the frequency and/or severity of tonic-clonic seizures, requiring an increase in the dosage of standard anticonvulsant medication. In such cases, abrupt withdrawal of chronic benzodiazepines may also be associated with a temporary increase in the frequency and/or severity of seizures.
10. Overdosage
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [see Warnings and Precautions (5.2)]. Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway maintenance. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting the Poison Help line (1-800-222-1222) or medical toxicologist for additional overdosage management recommendations.
11. Nayzilam Description
NAYZILAM contains midazolam, a compound of the benzodiazepine class. Midazolam is chemically designated as 8-Chloro-6-(ο-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine, and it has the following structure:
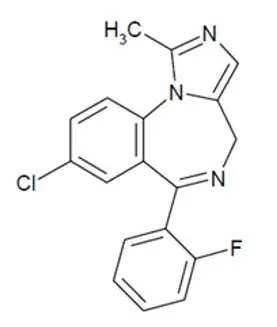
The empirical formula is C18H13ClFN3 representing a molecular weight of 325.8. Midazolam, USP is a white or yellowish, crystalline powder that is practically insoluble in water, soluble in methanol, and freely soluble in acetone and in alcohol.
NAYZILAM nasal spray is a clear, colorless to yellowish colored liquid. Each single-dose NAYZILAM unit is for nasal administration and delivers 5 mg of midazolam in 0.1 mL of solution containing ethanol; PEG-6 methyl ether; polyethylene glycol 400; propylene glycol; and purified water.
The pH range of solution is approximately 5.0 to 9.0.
12. Nayzilam - Clinical Pharmacology
12.1 Mechanism of Action
The exact mechanism of action for midazolam is not fully understood, but it is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABAA receptor.
12.2 Pharmacodynamics
The pharmacodynamic properties of midazolam and its metabolites, are similar to those of other benzodiazepines, including sedative, anxiolytic, amnestic, and hypnotic activities. The effects of midazolam on the CNS are dependent on the dose administered, the route of administration, and the presence or absence of other medications.
Treatment with NAYZILAM was associated with effects on measures of sedation and measures of psychomotor performance [see Warnings and Precautions (5.5)]. Sedation and psychomotor impairment effects generally began to occur within 10 minutes post dose with peak effects observed within 30 minutes to 2 hours post dose. The pharmacodynamic effects generally returned to near baseline levels by 4 hours post-dose.
12.3 Pharmacokinetics
14. Clinical Studies
The effectiveness of NAYZILAM for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy 12 years of age and older was established in a randomized, double-blind, placebo-controlled trial (Study 1; NCT 01390220).
Study 1 enrolled patients with epilepsy on a stable regimen of antiepileptic drugs who were identified by their physicians as having intermittent, stereotypic episodes of frequent seizure activity that were distinct from the patient's usual seizure pattern.
Study 1 was conducted in two phases: an open-label Test Dose Phase followed by a randomized, double-blind, placebo-controlled, Comparative Phase. In the Test Dose Phase, tolerability was assessed in 292 patients who, in the absence of a seizure, received two 5 mg doses of NAYZILAM (10 mg total dosage) separated by 10 minutes. Patients were excluded from participation in the Comparative Phase if they failed to meet pre-defined blood pressure, heart rate, sedation, electrocardiogram, and peripheral oxygen saturation criteria.
In the Comparative Phase, 201 patients treated a single seizure cluster episode in an outpatient setting with either a blinded dose of NAYZILAM 5 mg (134 patients) or placebo (67 patients). If the seizure activity persisted or recurred, patients in both groups had the option to receive a subsequent unblinded dose of NAYZILAM 5 mg to be used between 10 minutes and 6 hours after administration of the initial blinded dose of study drug.
The primary efficacy endpoint for Study 1 was treatment success, defined as the termination of seizures within 10 minutes after the initial blinded dose of study drug and the absence of a recurrence of seizures within 6 hours of the initial blinded dose of study drug. A statistically significantly higher percentage of NAYZILAM-treated patients met the primary efficacy endpoint, as shown in Table 4.
|
| NAYZILAM (N=134) | Placebo (N=67) |
|---|---|---|
| Treatment success (%) | 53.7 | 34.3 |
| 95% CI | (45.3, 62.2) | (23.0, 45.7) |
| p-value | 0.011 | |
Numerical differences in favor of NAYZILAM were observed on each of the components of the treatment success responder definition; termination of seizure(s) within 10 minutes after initial dose of study drug (80.6 versus 70.1%) and the absence of seizure recurrence between 10 minutes and 6 hours after the initial dose of study drug (58.2 versus 37.3%).
Study 1 also evaluated the occurrence and time to next seizure after the initial blinded dose of study drug. A smaller proportion of NAYZILAM-treated patients experienced the next seizure within 24 hours after the initial blinded dose of study drug (37.3% versus 46.3%). NAYZILAM-treated patients experienced a statistically longer time-to-next-seizure than the placebo group (Figure 1).
FIGURE 1: Kaplan-Meier Analysis of Time-to-Next-Seizure (Study 1)
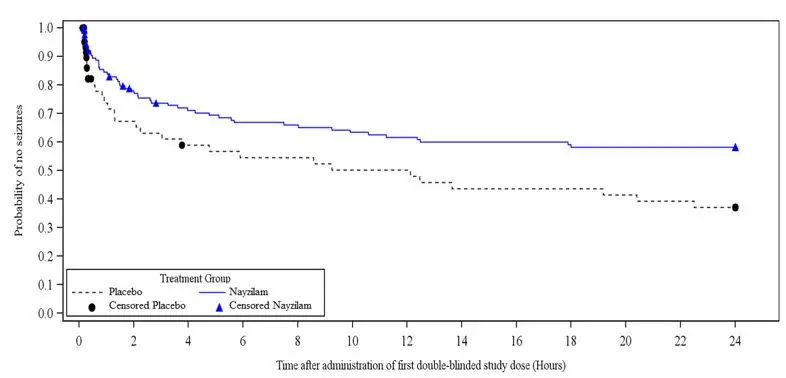
Analysis by gender revealed no substantial differences in treatment response. Informative subgroup analyses by age and race were not possible because of the small percentage of patients less than 18 years of age or 65 years of age or greater, and of non-White patients in the study.
16. How is Nayzilam supplied
17. Patient Counseling Information
Advise patients and caregivers to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
| MEDICATION GUIDE NAYZILAM® (NAY-zil-am) (midazolam) nasal spray, CIV |
||
|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Issued: 1/2023 | |
What is the most important information I should know about NAYZILAM?
|
||
|
|
|
How can I watch for early symptoms of suicidal thoughts or actions?
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes. |
||
What is NAYZILAM?
|
||
Do not use NAYZILAM if you:
|
||
Before you use NAYZILAM, tell your healthcare provider about all your medical conditions, including if you:
|
||
How should I use NAYZILAM?
|
||
| What should I avoid while using NAYZILAM? See "What is the most important information I should know about NAYZILAM?" |
||
| What are the possible side effects of NAYZILAM? NAYZILAM may cause serious side effects, including:
|
||
|
|
|
| These are not all the possible side effects of NAYZILAM. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
How should I store NAYZILAM?
|
||
| General information about the safe and effective use of NAYZILAM.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NAYZILAM for a condition for which it was not prescribed. Do not give NAYZILAM to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about NAYZILAM that is written for health professionals. |
||
| What are the ingredients in NAYZILAM?
Active ingredient: midazolam Inactive ingredients: ethanol, PEG-6 methyl ether, polyethylene glycol 400, propylene glycol and purified water Manufactured for UCB, Inc., Smyrna, GA 30080. NAYZILAM® is a registered trademark of the UCB Group of Companies. ©2022. All rights reserved. For more information, go to www.nayzilam.com or call 1-844-599-2273. |
||
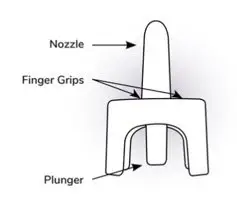
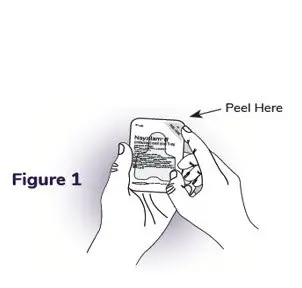
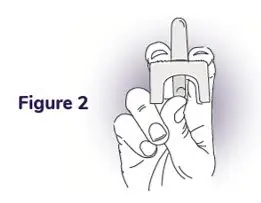
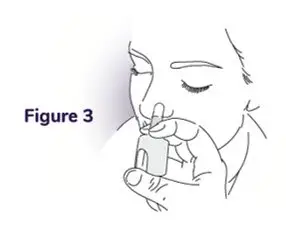
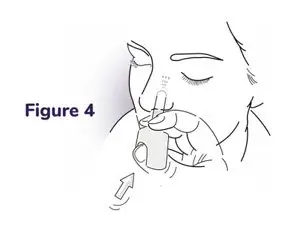
Instructions for Use
NAYZILAM® (NAY-zil-am)
(midazolam) nasal spray, CIV
You and your family members or caregivers should read this Instructions for Use before you start using NAYZILAM nasal spray and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. If you and your family members or caregivers have any questions about NAYZILAM, ask your healthcare provider or pharmacist.
|
|
|
| Local Emergency Number:________________________ |
| Healthcare Provider's Number:_______________________ |
| Information for Emergency Responder |
| Time of first NAYZILAM dose:________________ |
| Time of second NAYZILAM dose (if given):_________________________ | |
Manufactured for UCB, Inc., Smyrna, GA 30080.
NAYZILAM® is a registered trademark of the UCB Group of Companies. ©2022. All rights reserved.
For more information, go to www.nayzilam.com or call 1-844-599-2273.
This Instructions for Use has been approved by the U.S. Food and Drug Administration
Issued: 1/2023
| NAYZILAM
midazolam spray |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - UCB, Inc. (028526403) |
| Registrant - UCB, Inc. (043546522) |