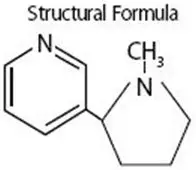
Drug Detail:Nicotrol inhaler (nasal, inhalation) (Nicotine (nasal, inhalation) [ nik-oh-teen ])
Drug Class: Smoking cessation agents
Nicotrol Inhalant - Clinical Pharmacology
Pharmacodynamics
The cardiovascular effects of nicotine include peripheral vasoconstriction, tachycardia, and elevated blood pressure. Acute and chronic tolerance to nicotine develops from smoking tobacco or ingesting nicotine preparations. Acute tolerance (a reduction in response for a given dose) develops rapidly (less than 1 hour), but not at the same rate for different physiologic effects (skin temperature, heart rate, subjective effects). Withdrawal symptoms such as cigarette craving can be reduced in most individuals by plasma nicotine levels lower than those from smoking.
Withdrawal from nicotine in addicted individuals can be characterized by craving, nervousness, restlessness, irritability, mood lability, anxiety, drowsiness, sleep disturbances, impaired concentration, increased appetite, minor somatic complaints (headache, myalgia, constipation, fatigue), and weight gain. Nicotine toxicity is characterized by nausea, abdominal pain, vomiting, diarrhea, diaphoresis, flushing, dizziness, disturbed hearing and vision, confusion, weakness, palpitations, altered respiration and hypotension.
Both smoking and nicotine can increase circulating cortisol and catecholamines, and tolerance does not develop to the catecholaminereleasing effects of nicotine. Changes in the response to a concomitantly administered adrenergic agonist or antagonist should be watched for when nicotine intake is altered during NICOTROL Inhaler therapy and/or smoking cessation (See PRECAUTIONS, Drug Interactions).
Warnings
Nicotine from any source can be toxic and addictive. Smoking causes lung disease, cancer and heart disease, and may adversely affect pregnant women or the fetus. For any smoker, with or without concomitant disease or pregnancy, the risk of nicotine replacement in a smoking cessation program should be weighed against the hazard of continued smoking, and the likelihood of achieving cessation of smoking without nicotine replacement.
Safety Note Concerning Children
This product contains nicotine and should be kept out of the reach of children and pets. The amounts of nicotine that are tolerated by adult smokers can produce signs and symptoms of poisoning and could prove fatal if the nicotine from the NICOTROL Inhaler is inhaled, ingested, or buccally absorbed by children or pets. Suspected nicotine poisoning in a child should be considered a medical emergency and treated immediately. A cartridge contains about 60% of its initial drug content when it is discarded, which is about 6 mg. Patients should be cautioned to keep both the used and unused cartridges of NICOTROL Inhaler out of the reach of children and pets.
All components of the NICOTROL Inhaler system should also be kept out of the reach of children and pets to avoid accidental swallowing and choking.
Precautions
Drug Interactions
Physiological changes resulting from smoking cessation, with or without nicotine replacement, may alter the pharmacokinetics of certain concomitant medications, such as tricyclic antidepressants and theophylline. Doses of these and perhaps other medications may need to be adjusted in patients who successfully quit smoking.
PREGNANCY
The harmful effects of cigarette smoking on maternal and fetal health are clearly established. These include low birth weight, an increased risk of spontaneous abortion, and increased perinatal mortality. The specific effects of NICOTROL Inhaler therapy on fetal development are unknown. Therefore pregnant smokers should be encouraged to attempt cessation using educational and behavioral interventions before using pharmacological approaches.
Spontaneous abortion during nicotine replacement therapy has been reported; as with smoking, nicotine as a contributing factor cannot be excluded.
NICOTROL Inhaler therapy should be used during pregnancy only if the likelihood of smoking cessation justifies the potential risk of using it by the pregnant patient, who might continue to smoke.
Adverse Reactions/Side Effects
Assessment of adverse events in the 1,439 patients (730 on active drug) who participated in controlled clinical trials (including three dose finding studies) is complicated by the occurrence of signs and symptoms of nicotine withdrawal in some patients and nicotine excess in others. The incidence of adverse events is confounded by: (1) the many minor complaints that smokers commonly have, (2) continued smoking by many patients and (3) the local irritation from both the active drug and the placebo.
Drug Abuse and Dependence
NICOTROL Inhaler is likely to have a low abuse potential based on differences between the product and cigarettes in three characteristics commonly considered important in contributing to abuse: slower absorption, smaller fluctuations in blood levels and lower blood levels of nicotine. NICOTROL Inhaler, like many other nicotine-based smoking cessation therapies, does not produce arterial concentrations similar to cigarettes. However, nicotine withdrawal symptoms were noted in clinical trials at the time of NICOTROL Inhaler tapering and after NICOTROL Inhaler discontinuation.
Dependence might occur from transference of tobacco-related nicotine dependence to the NICOTROL Inhaler. The use of the inhaler beyond 6 months has not been evaluated in clinical trials and is not recommended. To minimize the risk of dependence, patients should be encouraged to withdraw gradually from NICOTROL Inhaler therapy after 3 months of usage (See DOSAGE AND ADMINISTRATION). If necessary, dose reduction can be achieved by gradual reduction of the dose over a 6 to 12 week period.
Nicotrol Inhalant Dosage and Administration
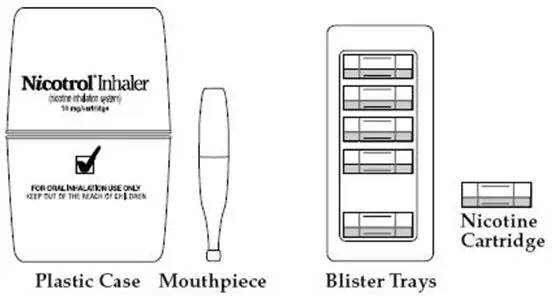
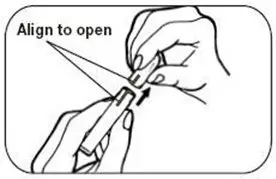
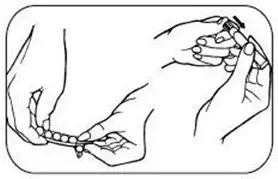
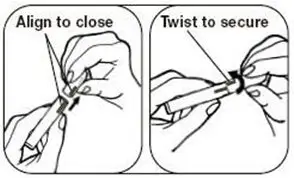
Patients must desire to stop smoking and should be instructed to stop smoking completely as they begin using NICOTROL Inhaler. It is important that patients understand the instructions, and have their questions answered. They should clearly understand the directions for using the NICOTROL Inhaler and safely disposing of the used cartridges.
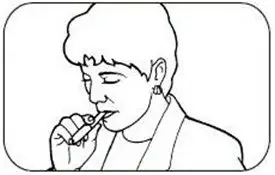
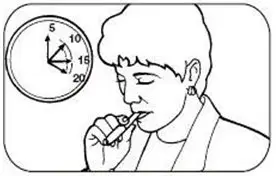
The initial dosage of NICOTROL Inhaler is individualized. Patients may selftitrate to the level of nicotine they require. Most successful patients in the clinical trials used between 6 and 16 cartridges a day. Best effect was achieved by frequent continuous puffing (20 minutes). The recommended duration of treatment is 3 months, after which patients may be weaned from the NICOTROL Inhaler by gradual reduction of the daily dose over the following 6 to 12 weeks. The safety and efficacy of the continued use of NICOTROL Inhaler for periods longer than 6 months have not been studied and such use is not recommended.
Dosing recommendations are summarized in the table below.
Individualization of Dosage
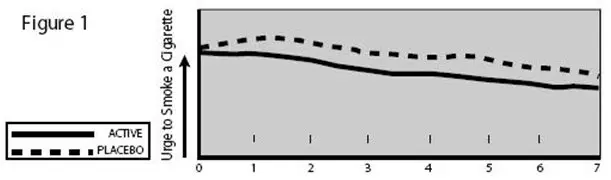
The NICOTROL Inhaler provides the smoker with adequate amounts of nicotine to reduce the urge to smoke, and may provide some degree of comfort by providing a hand-to-mouth ritual similar to smoking, although the importance of such an effect in smoking cessation is, as yet, unknown.
The success or failure of smoking cessation is influenced by the quality, intensity and frequency of supportive care. Patients are more likely to quit smoking if they are seen frequently and participate in formal smoking cessation programs.
The goal of NICOTROL Inhaler therapy is complete abstinence. If a patient is unable to stop smoking by the fourth week of therapy, treatment should probably be discontinued.
Patients who fail to quit on any attempt may benefit from interventions to improve their chances for success on subsequent attempts. Patients who were unsuccessful should be counseled and should then probably be given a therapeutic holiday before the next attempt. A new quit attempt should be encouraged when conditions are more favorable.
Based on the clinical trials, a reasonable approach to assisting patients in their attempt to quit smoking is to begin initial treatment, using the recommended dosage (See DOSAGE AND ADMINISTRATION). Dosage can then be adjusted in those patients with signs or symptoms of nicotine withdrawal or excess. Patients who are successfully abstinent on NICOTROL Inhaler should be treated at the selected dosage for up to 12 weeks, after which use of the Inhaler should be gradually reduced over the next 6 to 12 weeks. Some patients may not require gradual reduction of dosage and may abruptly stop treatment successfully. The safe use of this product for longer than 6 months has not been established.
The symptoms of nicotine withdrawal overlap those of nicotine excess (See Pharmacodynamics and ADVERSE REACTIONS sections). Since patients using NICOTROL Inhaler may also smoke intermittently, it is sometimes difficult to determine if they are experiencing nicotine withdrawal or nicotine excess. Controlled clinical trials of nicotine products suggest that palpitations, nausea and sweating are more often symptoms of nicotine excess, whereas anxiety, nervousness and irritability are more often symptoms of nicotine withdrawal.
| NICOTROL
nicotine inhalant |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc. | 053217022 | PACK(0009-5400) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 985052076 | ANALYSIS(0009-5400) , API MANUFACTURE(0009-5400) | |