Drug Detail:Ninlaro (Ixazomib [ ix-az-oh-mib ])
Drug Class: Proteasome inhibitors
Highlights of Prescribing Information
NINLARO® (ixazomib) capsules, for oral use
Initial U.S. Approval: 2015
Recent Major Changes
| Indications and Usage, Limitations of Use (1) | 4/2022 |
| Warnings and Precautions, Cutaneous Reactions (5.5) | 4/2022 |
| Warnings and Precautions, Increased Mortality in Patients Treated with NINLARO in the Maintenance Setting (5.9) | 4/2022 |
Indications and Usage for Ninlaro
NINLARO is a proteasome inhibitor indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy. (1)
Limitations of Use: NINLARO is not recommended for use in the maintenance setting or in newly diagnosed multiple myeloma in combination with lenalidomide and dexamethasone outside of controlled clinical trials. (1)
Ninlaro Dosage and Administration
- Recommended starting dose of 4 mg taken orally on Days 1, 8, and 15 of a 28-day cycle. (2.1)
- Dose should be taken at least one hour before or at least two hours after food. (2.1)
Dosage Forms and Strengths
Capsules: 4 mg, 3 mg, and 2.3 mg (3)
Contraindications
None. (4)
Warnings and Precautions
- Thrombocytopenia: Monitor platelet counts at least monthly during treatment and adjust dosing, as needed. (2.2, 5.1)
- Gastrointestinal Toxicities: Adjust dosing for severe diarrhea, constipation, nausea, and vomiting, as needed. (2.2, 5.2)
- Peripheral Neuropathy: Monitor patients for symptoms of peripheral neuropathy and adjust dosing, as needed. (2.2, 5.3)
- Peripheral Edema: Monitor for fluid retention. Investigate for underlying causes, when appropriate. Adjust dosing, as needed. (2.2, 5.4)
- Cutaneous Reactions: Monitor patients for rash and adjust dosing, as needed. (2.2, 5.5)
- Thrombotic Microangiopathy: Monitor for signs and symptoms. Discontinue NINLARO if suspected. (5.6)
- Hepatotoxicity: Monitor hepatic enzymes during treatment. (5.7)
- Embryo-Fetal Toxicity: NINLARO can cause fetal harm. Advise patients of the potential risk to a fetus and to use effective non-hormonal contraception. (5.8, 8.1, 8.3)
- Increased Mortality in Patients Treated with NINLARO in the Maintenance Setting: Treatment of patients with NINLARO for multiple myeloma in the maintenance setting is not recommended outside of controlled trials. (5.9)
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 20%) are thrombocytopenia, neutropenia, diarrhea, constipation, peripheral neuropathy, nausea, peripheral edema, rash, vomiting, and bronchitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals at 1-844-617-6468 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Strong CYP3A inducers: Avoid concomitant use with NINLARO. (7.1, 12.3)
Use In Specific Populations
- Hepatic Impairment: Reduce the NINLARO starting dose to 3 mg in patients with moderate or severe hepatic impairment. (2.3, 8.6)
- Renal Impairment: Reduce the NINLARO starting dose to 3 mg in patients with severe renal impairment or end-stage renal disease requiring dialysis. (2.4, 8.7)
- Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2022
Full Prescribing Information
1. Indications and Usage for Ninlaro
NINLARO is indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy.
2. Ninlaro Dosage and Administration
2.1 Dosing and Administration Guidelines
NINLARO in combination with lenalidomide and dexamethasone
The recommended starting dose of NINLARO is 4 mg administered orally once a week on Days 1, 8, and 15 of a 28-day treatment cycle.
The recommended starting dose of lenalidomide is 25 mg administered daily on Days 1 through 21 of a 28-day treatment cycle.
The recommended starting dose of dexamethasone is 40 mg administered on Days 1, 8, 15, and 22 of a 28-day treatment cycle.
| 28-Day Cycle (a 4-week cycle) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | |||||
| Day 1 | Days 2-7 | Day 8 | Days 9-14 | Day 15 | Days 16-21 | Day 22 | Days 23-28 | |
| NINLARO | ✔ | ✔ | ✔ | |||||
| Lenalidomide | ✔ | ✔ Daily | ✔ | ✔ Daily | ✔ | ✔ Daily | ||
| Dexamethasone | ✔ | ✔ | ✔ | ✔ | ||||
For additional information regarding lenalidomide and dexamethasone, refer to their prescribing information.
NINLARO should be taken once a week on the same day and at approximately the same time for the first three weeks of a four week cycle. The importance of carefully following all dosage instructions should be discussed with patients starting treatment. Instruct patients to take the recommended dosage as directed, because overdosage has led to deaths [see Overdosage (10)].
NINLARO should be taken at least one hour before or at least two hours after food [see Clinical Pharmacology (12.3)]. The whole capsule should be swallowed with water. The capsule should not be crushed, chewed or opened [see How Supplied/Storage and Handling (16)].
If a NINLARO dose is delayed or missed, the dose should be taken only if the next scheduled dose is ≥ 72 hours away. A missed dose should not be taken within 72 hours of the next scheduled dose. A double dose should not be taken to make up for the missed dose.
If vomiting occurs after taking a dose, the patient should not repeat the dose. The patient should resume dosing at the time of the next scheduled dose.
Prior to initiating a new cycle of therapy:
- Absolute neutrophil count should be at least 1,000/mm3
- Platelet count should be at least 75,000/mm3
- Non-hematologic toxicities should, at the healthcare provider's discretion, generally be recovered to patient's baseline condition or Grade 1 or lower
Treatment should be continued until disease progression or unacceptable toxicity.
2.2 Dosage Modification Guidelines
The NINLARO dose reduction steps are presented in Table 2 and the dosage modification guidelines are provided in Table 3.
|
|||
| Recommended starting dose* | First reduction to | Second reduction to | Discontinue |
| 4 mg | 3 mg | 2.3 mg | |
An alternating dose modification approach is recommended for NINLARO and lenalidomide for thrombocytopenia, neutropenia, and rash as described in Table 3. Refer to the lenalidomide prescribing information if dose reduction is needed for lenalidomide.
|
|
| Hematological Toxicities | Recommended Actions |
| Thrombocytopenia (Platelet Count) | |
| Platelet count less than 30,000/mm3 |
|
| Neutropenia (Absolute Neutrophil Count) | |
| Absolute neutrophil count less than 500/mm3 |
|
| Non-Hematological Toxicities | Recommended Actions |
| Rash | |
| Grade† 2 or 3 |
|
| Grade 4 | Discontinue treatment regimen. |
| Peripheral Neuropathy | |
| Grade 1 Peripheral Neuropathy with Pain or Grade 2 Peripheral Neuropathy |
|
| Grade 2 Peripheral Neuropathy with Pain or Grade 3 Peripheral Neuropathy |
|
| Grade 4 Peripheral Neuropathy | Discontinue treatment regimen. |
| Other Non-Hematological Toxicities | |
| Other Grade 3 or 4 Non-Hematological Toxicities |
|
2.3 Dosage in Patients with Hepatic Impairment
Reduce the starting dose of NINLARO to 3 mg in patients with moderate (total bilirubin greater than 1.5-3 × ULN) or severe (total bilirubin greater than 3 × ULN) hepatic impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.4 Dosage in Patients with Renal Impairment
Reduce the starting dose of NINLARO to 3 mg in patients with severe renal impairment (creatinine clearance less than 30 mL/min) or end-stage renal disease (ESRD) requiring dialysis. NINLARO is not dialyzable and therefore can be administered without regard to the timing of dialysis [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
Refer to the lenalidomide prescribing information for dosing recommendations in patients with renal impairment.
3. Dosage Forms and Strengths
NINLARO is available in the following capsules:
- 4 mg: Light orange gelatin capsule imprinted with "Takeda" on the cap and "4 mg" on the body in black ink.
- 3 mg: Light grey gelatin capsule imprinted with "Takeda" on the cap and "3 mg" on the body in black ink.
- 2.3 mg: Light pink gelatin capsule imprinted with "Takeda" on the cap and "2.3 mg" on the body in black ink.
5. Warnings and Precautions
5.1 Thrombocytopenia
Thrombocytopenia has been reported with NINLARO with platelet nadirs typically occurring between Days 14-21 of each 28-day cycle and recovery to baseline by the start of the next cycle [see Adverse Reactions (6.1)]. Grade 3 thrombocytopenia was reported in 17% of patients in the NINLARO regimen and Grade 4 thrombocytopenia was reported in 13% in the NINLARO regimen. The rate of platelet transfusions was 10% in the NINLARO regimen and 7% in the placebo regimen.
Monitor platelet counts at least monthly during treatment with NINLARO. Consider more frequent monitoring during the first three cycles. Manage thrombocytopenia with dose modifications [see Dosage and Administration (2.2)] and platelet transfusions as per standard medical guidelines.
5.2 Gastrointestinal Toxicities
Diarrhea, constipation, nausea, and vomiting have been reported with NINLARO, occasionally requiring use of antidiarrheal and antiemetic medications, and supportive care. Diarrhea was reported in 52% of patients in the NINLARO regimen and 43% in the placebo regimen, constipation in 35% and 28%, respectively, nausea in 32% and 23%, respectively, and vomiting in 26% and 13%, respectively. Diarrhea resulted in discontinuation of one or more of the three drugs in 3% of patients in the NINLARO regimen and 2% of patients in the placebo regimen [see Adverse Reactions (6.1)]. Adjust dosing for Grade 3 or 4 symptoms [see Dosage and Administration (2.2)].
5.3 Peripheral Neuropathy
The majority of peripheral neuropathy adverse reactions were Grade 1 (18% in the NINLARO regimen and 16% in the placebo regimen) and Grade 2 (11% in the NINLARO regimen and 6% in the placebo regimen) [see Adverse Reactions (6.1)]. Grade 3 adverse reactions of peripheral neuropathy were reported at 2% in both regimens.
The most commonly reported reaction was peripheral sensory neuropathy (24% and 17% in the NINLARO and placebo regimen, respectively). Peripheral motor neuropathy was not commonly reported in either regimen (< 1%). Peripheral neuropathy resulted in discontinuation of one or more of the three drugs in 4% of patients in the NINLARO regimen and <1% of patients in the placebo regimen. Patients should be monitored for symptoms of neuropathy. Patients experiencing new or worsening peripheral neuropathy may require dose modification [see Dosage and Administration (2.2)].
5.4 Peripheral Edema
Peripheral edema was reported in 27% and 21% of patients in the NINLARO and placebo regimens, respectively. The majority of peripheral edema adverse reactions were Grade 1 (17% in the NINLARO regimen and 14% in the placebo regimen) and Grade 2 (7% in the NINLARO regimen and 6% in the placebo regimen).
Grade 3 peripheral edema was reported in 2% and 1% of patients in the NINLARO and placebo regimens, respectively [see Adverse Reactions (6.1)]. Peripheral edema resulted in discontinuation of one or more of the three drugs in <1% of patients in both regimens. Evaluate for underlying causes and provide supportive care, as necessary. Adjust dosing of dexamethasone per its prescribing information or NINLARO for Grade 3 or 4 symptoms [see Dosage and Administration (2.2)].
5.5 Cutaneous Reactions
Rash was reported in 27% of patients in the NINLARO regimen and 16% of patients in the placebo regimen. The majority of the rash adverse reactions were Grade 1 (15% in the NINLARO regimen and 9% in the placebo regimen) or Grade 2 (9% in the NINLARO regimen and 4% in the placebo regimen) [see Adverse Reactions (6.1)]. Grade 3 rash was reported in 3% of patients in the NINLARO regimen and 2% of patients in the placebo regimen. Serious adverse reactions of rash were reported in <1% of patients in the NINLARO regimen. The most common type of rash reported in both regimens included maculo-papular and macular rash. Rash resulted in discontinuation of one or more of the three drugs in < 1% of patients in both regimens. Manage rash with supportive care or with dose modification if Grade 2 or higher [see Dosage and Administration (2.2)].
Stevens-Johnson syndrome, including a fatal case, has been reported with NINLARO [see Adverse Reactions (6.1)]. If Stevens-Johnson syndrome occurs, discontinue NINLARO and manage as clinically indicated.
5.6 Thrombotic Microangiopathy
Cases, sometimes fatal, of thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), have been reported in patients who received NINLARO [see Adverse Reactions (6.1)]. Monitor for signs and symptoms of TTP/HUS. If the diagnosis is suspected, stop NINLARO and evaluate. If the diagnosis of TTP/HUS is excluded, consider restarting NINLARO. The safety of reinitiating NINLARO therapy in patients previously experiencing TTP/HUS is not known.
5.7 Hepatotoxicity
Drug-induced liver injury, hepatocellular injury, hepatic steatosis, hepatitis cholestatic and hepatotoxicity have each been reported in < 1% of patients treated with NINLARO [see Adverse Reactions (6.1)]. Hepatotoxicity has been reported (10% in the NINLARO regimen and 9% in the placebo regimen). Monitor hepatic enzymes regularly and adjust dosing for Grade 3 or 4 symptoms [see Dosage and Administration (2.2)].
5.8 Embryo-Fetal Toxicity
NINLARO can cause fetal harm when administered to a pregnant woman based on the mechanism of action and findings in animal studies. Ixazomib caused embryo-fetal toxicity in pregnant rats and rabbits at doses resulting in exposures that were slightly higher than those observed in patients receiving the recommended dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective non-hormonal contraception during treatment with NINLARO and for 90 days following the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with NINLARO and for 90 days following the last dose [see Drug Interactions (7.1) and Use in Specific Populations (8.1, 8.3)].
5.9 Increased Mortality in Patients Treated with NINLARO in the Maintenance Setting
In two prospective randomized clinical trials in multiple myeloma in the maintenance setting, treatment with NINLARO resulted in increased deaths. Treatment of patients with NINLARO for multiple myeloma in the maintenance setting is not recommended outside of controlled trials [see Clinical Studies (14.2)].
6. Adverse Reactions/Side Effects
The following adverse reactions are described in detail in other sections of the prescribing information:
- Thrombocytopenia [see Warnings and Precautions (5.1)]
- Gastrointestinal Toxicities [see Warnings and Precautions (5.2)]
- Peripheral Neuropathy [see Warnings and Precautions (5.3)]
- Peripheral Edema [see Warnings and Precautions (5.4)]
- Cutaneous Reactions [see Warnings and Precautions (5.5)]
- Thrombotic Microangiopathy [see Warnings and Precautions (5.6)]
- Hepatotoxicity [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety population from the randomized, double-blind, placebo-controlled clinical study included 720 patients with relapsed and/or refractory multiple myeloma, who received NINLARO in combination with lenalidomide and dexamethasone (NINLARO regimen; N=361) or placebo in combination with lenalidomide and dexamethasone (placebo regimen; N=359).
The most frequently reported adverse reactions (≥ 20% with a difference of ≥5% compared to placebo) in the NINLARO regimen were thrombocytopenia, neutropenia, diarrhea, constipation, peripheral neuropathy, nausea, peripheral edema, rash, vomiting, and bronchitis. Serious adverse reactions reported in ≥ 2% of patients in the NINLARO regimen included diarrhea (3%), thrombocytopenia (2%) and bronchitis (2%). One or more of the three drugs was permanently discontinued in 4% of patients reporting peripheral neuropathy, 3% of patients reporting diarrhea and 2% of patients reporting thrombocytopenia. Permanent discontinuation of NINLARO due to an adverse reaction occurred in 10% of patients.
Table 4 summarizes the non-hematologic adverse reactions occurring in at least 5% of patients with at least a 5% difference between the NINLARO regimen and the placebo regimen.
| System Organ Class / Preferred Term | NINLARO + Lenalidomide and Dexamethasone N=361 | Placebo + Lenalidomide and Dexamethasone N=359 |
||||
|---|---|---|---|---|---|---|
| % | % | |||||
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | |
| Note: Adverse reactions included as preferred terms are based on MedDRA version 23.0. | ||||||
|
||||||
| Gastrointestinal disorders | ||||||
| Diarrhea | 52 | 10 | 0 | 43 | 3 | 0 |
| Constipation | 35 | < 1 | 0 | 28 | < 1 | 0 |
| Nausea | 32 | 2 | 0 | 23 | 0 | 0 |
| Vomiting | 26 | 1 | 0 | 13 | <1 | 0 |
| Nervous system disorders | ||||||
| Peripheral neuropathies* | 32 | 2 | 0 | 24 | 2 | 0 |
| Musculoskeletal and connective tissue disorders | ||||||
| Back pain† | 27 | <1 | 0 | 24 | 3 | 0 |
| Infections and infestations | ||||||
| Upper respiratory tract infection† | 27 | 1 | 0 | 23 | 1 | 0 |
| Bronchitis | 22 | 2 | 0 | 17 | 2 | <1 |
| Skin and subcutaneous tissue disorders | ||||||
| Rash* | 27 | 3 | 0 | 16 | 2 | 0 |
| General disorders and administration site conditions | ||||||
| Edema peripheral | 27 | 2 | 0 | 21 | 1 | 0 |
Table 5 represents pooled information from adverse event and laboratory data.
| NINLARO + Lenalidomide and Dexamethasone N=361 | Placebo + Lenalidomide and Dexamethasone N=359 |
|||
|---|---|---|---|---|
| % | % | |||
| Any Grade | Grade 3-4 | Any Grade | Grade 3-4 | |
| Thrombocytopenia | 85 | 30 | 67 | 14 |
| Neutropenia | 74 | 34 | 70 | 37 |
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
NINLARO can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
8.5 Geriatric Use
Of the total number of subjects in clinical studies of NINLARO, 55% were 65 and over, while 17% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
In patients with moderate or severe hepatic impairment, the mean AUC increased by 20% when compared to patients with normal hepatic function. Reduce the starting dose of NINLARO in patients with moderate or severe hepatic impairment [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
8.7 Renal Impairment
In patients with severe renal impairment or ESRD requiring dialysis, the mean AUC increased by 39% when compared to patients with normal renal function. Reduce the starting dose of NINLARO in patients with severe renal impairment or ESRD requiring dialysis. NINLARO is not dialyzable and therefore can be administered without regard to the timing of dialysis [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
10. Overdosage
Overdosage, including fatal overdosage, has been reported in patients taking NINLARO. Manifestations of overdosage include adverse reactions reported at the recommended dosage [see Dosage and Administration (2.1), Adverse Reactions (6.1)]. Serious adverse reactions reported with overdosage include severe nausea, vomiting, diarrhea, aspiration pneumonia, multiple organ failure and death.
In the event of an overdosage, monitor for adverse reactions and provide appropriate supportive care. NINLARO is not dialyzable.
11. Ninlaro Description
Ixazomib is a proteasome inhibitor. Ixazomib citrate, a prodrug, rapidly hydrolyzes under physiological conditions to its biologically active form, ixazomib. The chemical name of ixazomib citrate is 1,3,2-dioxaborolane-4,4-diacetic acid, 2-[(1R)-1-[[2-[(2,5-dichlorobenzoyl)amino]acetyl]amino]-3-methylbutyl]-5-oxo- and the structural formula is:
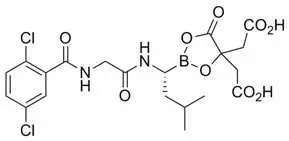
The molecular formula for ixazomib citrate is C20H23BCl2N2O9 and its molecular weight is 517.12. Ixazomib citrate has one chiral center and is the R-stereoisomer. The solubility of ixazomib citrate in 0.1N HCl (pH 1.2) at 37°C is 0.61 mg/mL (reported as ixazomib). The solubility increases as the pH increases.
NINLARO (ixazomib) capsules for oral use contain 4, 3 or 2.3 mg of ixazomib equivalent to 5.7, 4.3 or 3.3 mg of ixazomib citrate, respectively. Inactive ingredients include microcrystalline cellulose, magnesium stearate, and talc. Capsule shells contain gelatin and titanium dioxide. The 4 mg capsule shell contains red and yellow iron oxide, the 3 mg capsule shell contains black iron oxide and the 2.3 mg capsule shell contains red iron oxide. The printing ink contains shellac, propylene glycol, potassium hydroxide, and black iron oxide.
12. Ninlaro - Clinical Pharmacology
12.1 Mechanism of Action
Ixazomib is a reversible proteasome inhibitor. Ixazomib preferentially binds and inhibits the chymotrypsin-like activity of the beta 5 subunit of the 20S proteasome.
Ixazomib induced apoptosis of multiple myeloma cell lines in vitro. Ixazomib demonstrated in vitro cytotoxicity against myeloma cells from patients who had relapsed after multiple prior therapies, including bortezomib, lenalidomide, and dexamethasone. The combination of ixazomib and lenalidomide demonstrated synergistic cytotoxic effects in multiple myeloma cell lines. In vivo, ixazomib demonstrated antitumor activity in a mouse multiple myeloma tumor xenograft model.
12.3 Pharmacokinetics
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ixazomib was not mutagenic in a bacterial reverse mutation assay (Ames assay). Ixazomib was considered positive in an in vitro clastogenicity test in human peripheral blood lymphocytes. However, in vivo, ixazomib was not clastogenic in a bone marrow micronucleus assay in mice and was negative in an in vivo comet assay in mice, as assessed in the stomach and liver. No carcinogenicity studies have been performed with ixazomib.
Developmental toxicity studies in rats and rabbits did not show direct embryo-fetal toxicity below maternally toxic doses of ixazomib. Studies of fertility and early embryonic development and pre- and postnatal toxicology were not conducted with ixazomib, but evaluation of reproductive tissues was conducted in the general toxicity studies. There were no effects due to ixazomib treatment on male or female reproductive organs in studies up to 6-months duration in rats and up to 9-months duration in dogs.
14. Clinical Studies
14.1 Multiple Myeloma in Patients Who Have Received at Least One Prior Therapy
The efficacy and safety of NINLARO in combination with lenalidomide and dexamethasone was evaluated in a randomized, double-blind, placebo-controlled, multicenter study in patients with relapsed and/or refractory multiple myeloma who had received at least one prior line of therapy. Patients who were refractory to lenalidomide or proteasome inhibitors were excluded from the study.
A total of 722 patients were randomized in a 1:1 ratio to receive either the combination of NINLARO, lenalidomide and dexamethasone (N=360; NINLARO regimen) or the combination of placebo, lenalidomide and dexamethasone (N=362; placebo regimen) until disease progression or unacceptable toxicity. Randomization was stratified according to number of prior lines of therapy (1 versus 2 or 3), myeloma International Staging System (ISS) (stage I or II versus III), and previous therapy with a proteasome inhibitor (exposed or naïve). Twenty three percent (N=166) of the patients had light chain disease and 12% (N=87) of patients had free light chain-measurable only disease.
Thromboprophylaxis was recommended for all patients in both treatment groups according to the lenalidomide prescribing information. Antiemetics were used in 19% of patients in the NINLARO regimen and 12% of patients in the placebo regimen; antivirals in 64% and 60%, respectively, and antihistamines in 27% and 19%, respectively. These medications were given to patients at the healthcare provider's discretion as prophylaxis and/or management of symptoms.
Patients received NINLARO 4 mg or placebo on Days 1, 8, and 15 plus lenalidomide (25 mg) on Days 1 through 21 and dexamethasone (40 mg) on Days 1, 8, 15, and 22 of a 28-day cycle. Patients with renal impairment received a starting dose of lenalidomide according to its prescribing information. Treatment continued until disease progression or unacceptable toxicities.
Table 6 summarizes the baseline patient and disease characteristics in the study. The baseline demographics and disease characteristics were balanced and comparable between the study regimens.
| NINLARO + Lenalidomide and Dexamethasone (N = 360) | Placebo + Lenalidomide and Dexamethasone (N = 362) |
|
|---|---|---|
|
||
| Patient Characteristics | ||
| Median age in years (range) | 66 (38, 91) | 66 (30, 89) |
| Gender (%) Male/ Female | 58/42 | 56/44 |
| Age Group (% [< 65/ ≥ 65 years]) | 41/59 | 43/57 |
| Race n (%) | ||
| White | 310 (86) | 301 (83) |
| Black | 7 (2) | 6 (2) |
| Asian | 30 (8) | 34 (9) |
| Other or Not Specified | 13 (4) | 21 (6) |
| ECOG performance status, n (%) | ||
| 0 or 1 | 336 (93) | 334 (92) |
| 2 | 18 (5) | 24 (7) |
| Missing | 6 (2) | 4 (1) |
| Creatinine clearance, n (%) | ||
| < 30 mL/min | 5 (1) | 5 (1) |
| 30-59 mL/min | 74 (21) | 95 (26) |
| ≥ 60 mL/min | 281 (78) | 261 (72) |
| Disease Characteristics | ||
| Myeloma ISS stage, n (%) | ||
| Stage I or II | 315 (87) | 320 (88) |
| Stage III | 45 (13) | 42 (12) |
| Prior line therapies n (%) | ||
| Median (range) | 1 (1, 3) | 1 (1,3) |
| 1 | 224 (62) | 217 (60) |
| 2 or 3 | 136 (38) | 145 (40) |
| Status at Baseline n (%) | ||
| Relapsed | 276 (77) | 280 (77) |
| Refractory* | 42 (12) | 40 (11) |
| Relapsed and Refractory | 41 (11) | 42 (12) |
| Type of Prior Therapy n (%) | ||
| Bortezomib containing | 248 (69) | 250 (69) |
| Carfilzomib containing | 1 (<1) | 4 (1) |
| Thalidomide containing | 157 (44) | 170 (47) |
| Lenalidomide containing | 44 (12) | 44 (12) |
| Melphalan containing | 293 (81) | 291 (80) |
| Stem cell transplantation | 212 (59) | 199 (55) |
| High risk (deletion (del) 17, t(4:14) and/or t(14:16) | 75 (21) | 62 (17) |
| deletion del (17) | 36 (10) | 33 (9) |
The efficacy of NINLARO was evaluated by progression-free survival (PFS) according to the 2011 International Myeloma Working Group (IMWG) Consensus Uniform Response Criteria as assessed by a blinded independent review committee (IRC) based on central lab results. Response was assessed every four weeks until disease progression.
The approval of NINLARO was based upon a statistically significant improvement in PFS of the NINLARO regimen compared to the placebo regimen. PFS results are summarized in Table 7 and shown in Figure 1.
| NINLARO + Lenalidomide and Dexamethasone (N = 360) | Placebo + Lenalidomide and Dexamethasone (N = 362) |
|
|---|---|---|
| NE: Not evaluable. | ||
|
||
| Progression-free Survival | ||
| PFS Events, n (%) | 129 (36) | 157 (43) |
| Median (months) (95% CI) | 20.6 (17.0, NE) | 14.7 (12.9, 17.6) |
| Hazard Ratio*
(95% CI) | 0.74 (0.59, 0.94) |
|
| p-value† | 0.012 | |
| Response Rate | ||
| Overall Response Rate, n (%) | 282 (78) | 259 (72) |
| Complete Response | 42 (12) | 24 (7) |
| Very Good Partial Response | 131 (36) | 117 (32) |
| Partial Response | 109 (30) | 118 (33) |
The median time to response was 1.1 months in the NINLARO regimen and 1.9 months in the placebo regimen. The median duration of response was 20.5 months in the NINLARO regimen and 15 months in the placebo regimen for responders in the response evaluable population.
Figure 1: Kaplan-Meier Plot of Progression-Free Survival
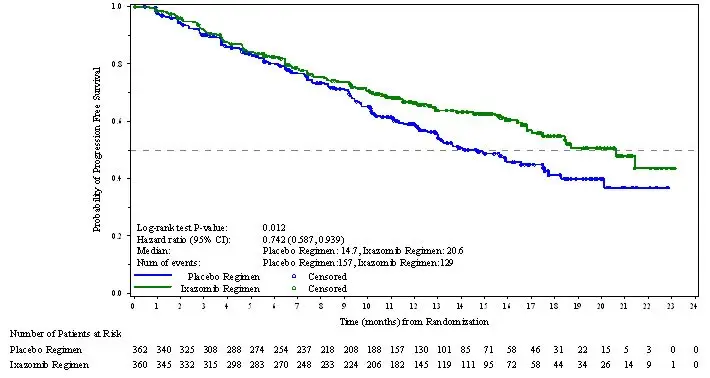
A non-inferential PFS analysis was conducted at a median follow up of 23 months with 372 PFS events. Hazard ratio of PFS was 0.82 (95% confidence interval [0.67, 1.0]) for NINLARO regimen versus placebo regimen, and estimated median PFS was 20 months in the NINLARO regimen and 15.9 months in the placebo regimen.
At the final analysis for OS at a median duration of follow up of approximately 85 months, median OS in the ITT population was 53.6 months for patients in the NINLARO regimen and 51.6 months for patients in the placebo regimen (HR = 0.94 [95% CI: 0.78, 1.13]).
14.2 Increased Mortality in Patients Treated with NINLARO in the Maintenance Setting
In C16019 (NCT02181413), newly diagnosed multiple myeloma patients who underwent autologous stem cell transplantation, continued on maintenance therapy for 24 months. There were 27% (105/395) deaths in the NINLARO arm compared with 26% (69/261) in the placebo arm. The hazard ratio for overall survival was 1.008 (95% CI: 0.744 - 1.367).
In C16021 (NCT02312258), newly diagnosed multiple myeloma patients, not treated with a stem cell transplant who achieved a partial response or better, continued on maintenance therapy for 24 months. There were 30% (127/425) deaths in the NINLARO arm compared with 27% (76/281) in the placebo arm. The hazard ratio for overall survival was 1.136 (95% CI: 0.853 - 1.514).
NINLARO is not recommended for use in the maintenance setting for multiple myeloma outside of controlled clinical trials [see Indications and Usage (1) and Warnings and Precautions (5.9)].
14.3 Lack of Efficacy in Patients with Newly Diagnosed Multiple Myeloma
Lack of efficacy in patients with newly diagnosed multiple myeloma was determined in a prospective randomized clinical trial.
In C16014 (NCT01850524), in newly diagnosed multiple myeloma patients, the study did not meet the prespecified primary endpoint for PFS. There were 136 (39%) deaths in the NINLARO, lenalidomide, and dexamethasone arm compared to 148 (42%) in the lenalidomide and dexamethasone arm. The hazard ratio for overall survival was 0.998 (95% CI: 0.79 - 1.261).
NINLARO is not recommended for use in combination with lenalidomide and dexamethasone in newly diagnosed multiple myeloma outside of controlled clinical trials [see Indications and Usage (1)].
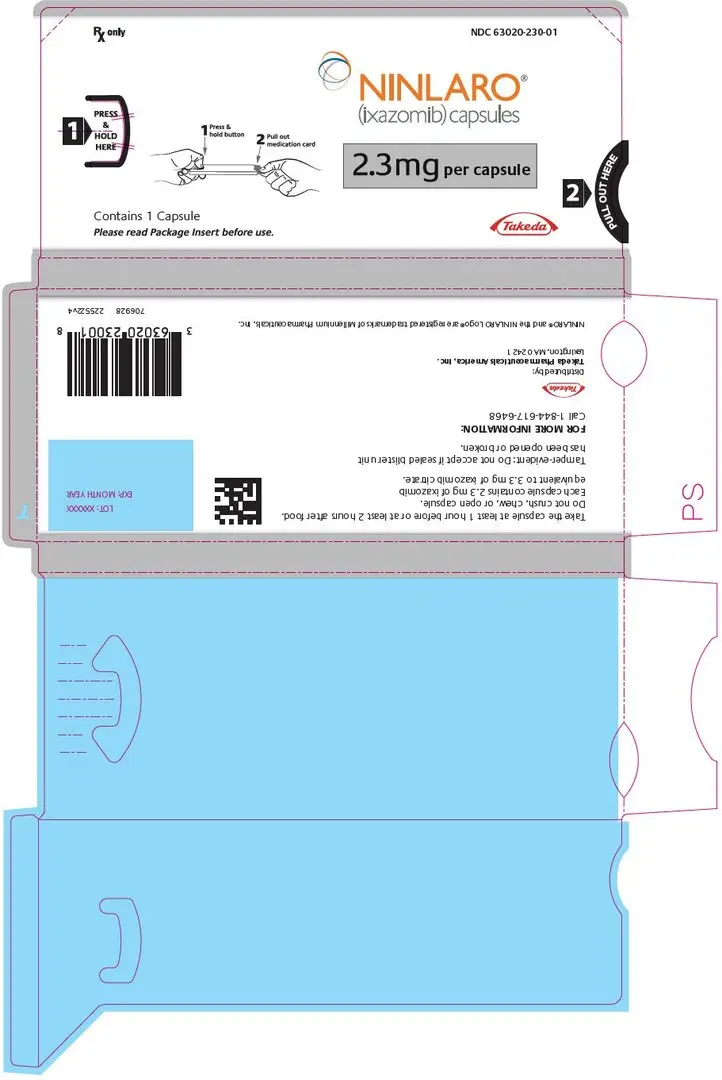
16. How is Ninlaro supplied
How Supplied
NINLARO is supplied as follows:
| Strength per Capsule | Capsule Description | Outer Carton | Blister Pack | NDC |
|---|---|---|---|---|
| 4 mg | Light orange, size 3, imprinted with "Takeda" on the cap and "4 mg" on the body in black ink. | Three 4 mg single blister packs in a carton | Each blister has one 4 mg capsule | Outer carton NDC 63020-400-02 Blister pack NDC 63020-400-01 |
| 3 mg | Light grey, size 4, imprinted with "Takeda" on the cap and "3 mg" on the body in black ink. | Three 3 mg single blister packs in a carton | Each blister has one 3 mg capsule | Outer carton NDC 63020-390-02 Blister pack NDC 63020-390-01 |
| 2.3 mg | Light pink, size 4, imprinted with "Takeda" on the cap and "2.3 mg" on the body in black ink. | Three 2.3 mg single blister packs in a carton | Each blister has one 2.3 mg capsule | Outer carton NDC 63020-230-02 Blister pack NDC 63020-230-01 |
Capsules are individually packaged in a PVC-Aluminum/Aluminum blister.
Storage
Store NINLARO at room temperature. Do not store above 30°C (86°F). Do not freeze.
Store capsules in original packaging until immediately prior to use.
Handling and Disposal
NINLARO is a hazardous drug. Follow applicable special handling and disposal procedures1.
Do not open or crush capsules. Avoid direct contact with the capsule contents. In case of capsule breakage, avoid direct contact of capsule contents with the skin or eyes. If contact occurs with the skin, wash thoroughly with soap and water. If contact occurs with the eyes, flush thoroughly with water.
Any unused medicinal product or waste material should be disposed in accordance with local requirements.
| NINLARO
ixazomib capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NINLARO
ixazomib capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NINLARO
ixazomib capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ash Stevens LLC | 049265333 | API MANUFACTURE(63020-230, 63020-390, 63020-400) , ANALYSIS(63020-230, 63020-390, 63020-400) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Curia Indiana, LLC. | 020593403 | ANALYSIS(63020-230, 63020-390, 63020-400) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Lancaster Laboratories, Inc | 069777290 | ANALYSIS(63020-400, 63020-390, 63020-230) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Haupt Pharma Amareg GmbH | 331334909 | MANUFACTURE(63020-400, 63020-390, 63020-230) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon (UK) Limited | 762771269 | PACK(63020-400, 63020-390, 63020-230) , LABEL(63020-400, 63020-390, 63020-230) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Takeda Ireland Ltd. | 986017670 | MANUFACTURE(63020-230, 63020-390, 63020-400) , PACK(63020-400, 63020-390, 63020-230) , LABEL(63020-400, 63020-390, 63020-230) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Biopharma Product Testing Ireland Limited | 238239933 | ANALYSIS(63020-400, 63020-390, 63020-230) | |