Drug Detail:Nithiodote (Sodium nitrite and sodium thiosulfate [ soo-dee-um-nye-trite-and-soo-de-um-thye-oh-sul-fate ])
Drug Class: Antidotes
Highlights of Prescribing Information
NITHIODOTE (sodium nitrite injection and sodium thiosulfate injection for intravenous infusion).
Initial U.S. Approval: 1992
WARNING: LIFE-THREATENING HYPOTENSION AND METHEMOGLOBIN FORMATION
See full prescribing information for complete boxed warning.
Sodium nitrite can cause serious adverse reactions and death from:
- Hypotension (5.1)
- Methemoglobin formation (5.2)
Patients should be closely monitored to ensure adequate perfusion and oxygenation during treatment with sodium nitrite.
Indications and Usage for Nithiodote
NITHIODOTE, an antidote, is indicated for the treatment of acute cyanide poisoning that is judged to be serious or life-threatening. (1)
- Use with caution if the diagnosis of cyanide poisoning is uncertain. (1)
Nithiodote Dosage and Administration
- If clinical suspicion of cyanide poisoning is high, administer NITHIODOTE without delay and in conjunction with appropriate airway, ventilatory, and circulatory support. (2.1)
- The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222. (2.1)
Dosing:
| Age | Intravenous Dose of Sodium Nitrite and Sodium Thiosulfate |
|---|---|
| Adults |
|
| Children |
|
- Redosing: If signs of cyanide poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate. (2.2)
- Monitoring: Blood pressure must be monitored during treatment. (2.2)
- NITHIODOTE is chemically incompatible with hydroxocobalamin and should not be administered via the same intravenous line. (2.4)
Dosage Forms and Strengths
NITHIODOTE consists of:
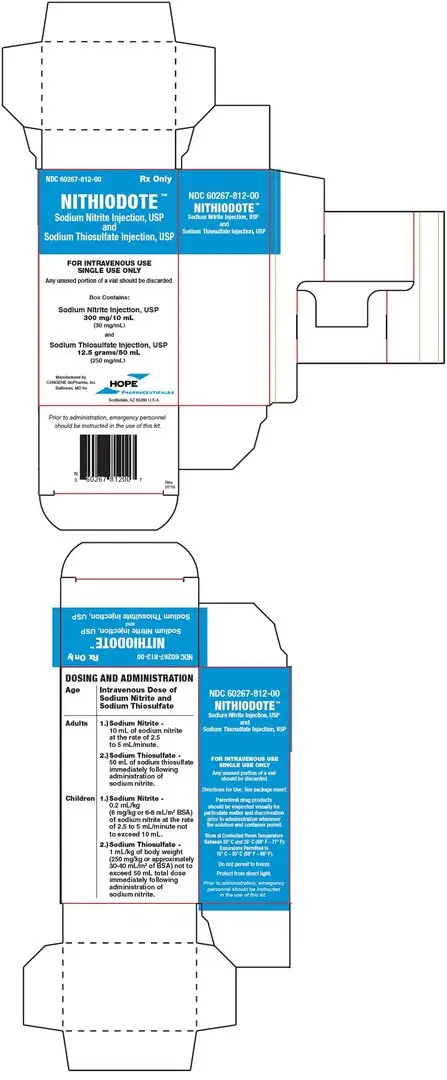
- one vial of sodium nitrite injection, USP 300 mg/10 mL (30 mg/mL) and
- one vial of sodium thiosulfate injection, USP 12.5 grams/50 mL (250 mg/mL). (3)
Contraindications
- None. (4)
Warnings and Precautions
- Methemoglobinemia: Sodium nitrite reacts with hemoglobin to form methemoglobin and should be used with caution in patients known to have anemia. Monitor oxyhemoglobin and methemoglobin levels by pulse co-oximetry or other measurements. Optimally, the sodium nitrite dose should be reduced in proportion to the oxygen carrying capacity. (5.2)
-
Smoke inhalation: Carbon monoxide contained in smoke can result in the formation of carboxyhemoglobin that can reduce the oxygen carrying capacity of the blood. Sodium nitrite should be used with caution in patients with smoke inhalation injury because of the potential for worsening hypoxia due to methemoglobin formation.
Carboxyhemoglobin and oxyhemoglobin levels should be monitored by pulse oximetry or other measurements in patients that present with evidence of smoke inhalation. Optimally, the sodium nitrite dose should be reduced in proportion to the oxygen carrying capacity. (5.4)
Adverse Reactions/Side Effects
Most common adverse reactions are:
- Sodium nitrite: syncope, hypotension, tachycardia, palpitations, dysrhythmia, methemoglobinemia, headache, dizziness, blurred vision, seizures, confusion, coma (6)
- Sodium thiosulfate: hypotension, headache, disorientation (6)
To report SUSPECTED ADVERSE REACTIONS, contact Hope Pharmaceuticals at 1-800-755-9595 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Lactation: Breastfeeding not recommended. (8.2)
- Renal impairment: Sodium nitrite and sodium thiosulfate are substantially excreted by the kidney. The risk of toxic reactions to these drugs may be greater in patients with impaired renal function. (8.6).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2021
Related/similar drugs
hydroxocobalamin, amyl nitrite, sodium thiosulfate, CyanokitFull Prescribing Information
WARNING: LIFE THREATENING HYPOTENSION AND METHEMOGLOBIN FORMATION
Sodium nitrite can cause serious adverse reactions and death in humans, even at doses less than twice the recommended therapeutic dose. Sodium nitrite causes hypotension and methemoglobin formation, which diminishes oxygen carrying capacity. Hypotension and methemoglobin formation can occur concurrently or separately. Because of these risks, sodium nitrite should be used to treat acute life-threatening cyanide poisoning and be used with caution in patients where the diagnosis of cyanide poisoning is uncertain.
Patients should be closely monitored to ensure adequate perfusion and oxygenation during treatment with sodium nitrite.
Alternative therapeutic approaches should be considered in patients known to have diminished oxygen or cardiovascular reserve (e.g. smoke inhalation victims, pre-existing anemia, cardiac or respiratory compromise), and those at higher risk of developing methemoglobinemia (e.g., congenital methemoglobin reductase deficiency) as they are at greater risk for potentially life-threatening adverse events related to the use of sodium nitrite. [See Warnings and Precautions (5.1 and 5.2)]
1. Indications and Usage for Nithiodote
NITHIODOTE is indicated for the treatment of acute cyanide poisoning that is judged to be serious or life-threatening. When the diagnosis of cyanide poisoning is uncertain, carefully weigh the potentially life-threatening risks associated with NITHIODOTE against the potential benefits, especially if the patient is not in extremis.
2. Nithiodote Dosage and Administration
2.1 Important Dosage and Administration Instructions
- If clinical suspicion of cyanide poisoning is high, administer NITHIODOTE without delay.
- Comprehensive treatment of acute cyanide intoxication requires support of vital functions. Administration of sodium nitrite and sodium thiosulfate should be considered adjunctive to appropriate supportive therapies. Airway, ventilatory and circulatory support, and oxygen administration should not be delayed in order to administer sodium nitrite and sodium thiosulfate [see Warnings and Precautions (5.1)].
- The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222.
2.2 Recommended Dosing
Sodium nitrite injection and sodium thiosulfate injection are administered by slow intravenous injection. They should be given as early as possible after a diagnosis of acute serious or life-threatening cyanide poisoning has been established. Sodium nitrite should be administered first, followed immediately by sodium thiosulfate. Blood pressure must be monitored during infusion in both adults and children. The rate of infusion should be decreased if significant hypotension is noted.
| Age | Intravenous Dose of Sodium Nitrite and Sodium Thiosulfate |
|---|---|
| Adults |
|
| Children |
|
NOTE: If signs of poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate.
In adult and pediatric patients with known anemia, it is recommended that the dosage of sodium nitrite should be reduced proportionately to the hemoglobin concentration. [see Warnings and Precautions (5.2)]
Visually inspect all parenteral drug products for particulate matter and discoloration prior to administration.
2.3 Recommended Monitoring
Monitor patients for at least 24-48 hours after NITHIODOTE administration for adequacy of oxygenation and perfusion and for recurrent signs and symptoms of cyanide toxicity. When possible, obtain hemoglobin/hematocrit when treatment is initiated. Measurements of oxygen saturation using standard pulse oximetry and calculated oxygen saturation values based on measured PO2 are unreliable in the presence of methemoglobinemia.
2.4 Incompatibility Information
Chemical incompatibility has been reported between NITHIODOTE and hydroxocobalamin and these drugs should not be administered simultaneously through the same IV line. No chemical incompatibility has been reported between sodium thiosulfate and sodium nitrite, when administered sequentially through the same IV line as described in Dosage and Administration.
Simultaneous administration of NITHIODOTE and blood products (whole blood, packed red cells, platelet concentrate and/or fresh frozen plasma) through the same intravenous line is not recommended. However, blood products and NITHIODOTE can be administered simultaneously using separate intravenous lines (preferably on contralateral extremities, if peripheral lines are being used).
3. Dosage Forms and Strengths
NITHIODOTE Injection consists of:
- One vial of sodium nitrite injection, USP 300 mg/10 mL (30 mg/mL) and
- One vial of sodium thiosulfate injection USP 12.5 grams/50 mL (250 mg/mL)
Administration of one vial of each medication constitutes a single dose.
5. Warnings and Precautions
5.1 Hypotension
Sodium nitrite has been associated with severe hypotension, methemoglobinemia, and death at doses less than twice recommended therapeutic doses. Hypotension may occur concurrently or separately. Sodium nitrite should be used to treat life-threatening cyanide poisoning. When the diagnosis of cyanide poisoning is uncertain and/or the patient is not in extremis, special consideration should be given to administration of sodium nitrite if the patient is known or suspected to have diminished oxygen or cardiovascular reserve (e.g., smoke inhalation victims, pre-existing anemia, substantial blood loss, cardiac or respiratory compromise) or to be at higher risk of developing methemoglobinemia (e.g., congenital methemoglobin reductase deficiency).
5.2 Methemoglobinemia
Supportive care alone may be sufficient treatment without administration of antidotes for many cases of cyanide intoxication, particularly in conscious patients without signs of severe toxicity. Monitor patients closely to ensure adequate perfusion and oxygenation during treatment with sodium nitrite.
Monitor methemoglobin levels and administer oxygen during treatment with sodium nitrite whenever possible. When sodium nitrite is administered to humans a wide range of methemoglobin concentrations occur. Methemoglobin concentrations as high as 58% have been reported after two 300-mg doses of sodium nitrite administered to an adult. Sodium nitrite should be used with caution in the presence of other drugs that may cause methemoglobinemia such as procaine and nitroprusside. Use sodium nitrite with caution in patients who may be particularly susceptible to injury from vasodilation and its related hemodynamic sequelae. Monitor hemodynamics closely during and after administration of sodium nitrite and sodium thiosulfate, and reduce infusion rates if hypotension occurs.
5.3 Anemia
Use sodium nitrite with caution in patients with known anemia. Patients with anemia will form more methemoglobin (as a percentage of total hemoglobin) than persons with normal red blood cell (RBC) volumes. Optimally, these patients should receive a sodium nitrite dose that is reduced in proportion to their oxygen carrying capacity.
5.4 Smoke Inhalation Injury
Use sodium nitrite with caution in persons with smoke inhalation injury or carbon monoxide poisoning because of the potential for worsening hypoxia due to methemoglobin formation.
5.5 Neonates and Infants
Neonates and infants may be more susceptible than adults and older pediatric patients to severe methemoglobinemia when sodium nitrite is administered. Follow reduced dosing guidelines in pediatric patients.
5.6 G6PD Deficiency
Because patients with G6PD deficiency are at increased risk of a hemolytic crisis with sodium nitrite administration, consider alternative therapeutic approaches in these patients. Monitor patients with known or suspected G6PD deficiency for an acute drop in hematocrit. Exchange transfusion may be needed for patients with G6PD deficiency who receive sodium nitrite.
6. Adverse Reactions/Side Effects
There have been no controlled clinical trials conducted to systematically assess the adverse events profile of sodium nitrite or sodium thiosulfate.
The medical literature has reported the following adverse events in association with sodium nitrite or sodium thiosulfate administration. These adverse events were not reported in the context of controlled trials or with consistent monitoring and reporting methodologies for adverse events. Therefore, frequency of occurrence of these adverse events cannot be assessed.
8. Use In Specific Populations
8.4 Pediatric Use
There are case reports in the medical literature of sodium nitrite in conjunction with sodium thiosulfate being administered to pediatric patients with cyanide poisoning; however, there have been no clinical studies to evaluate the safety or efficacy of sodium thiosulfate or sodium nitrite in the pediatric population. As for adult patients, dosing recommendations for pediatric patients have been based on theoretical calculations of antidote detoxifying potential, extrapolation from animal experiments, and a small number of human case reports.
Use Sodium nitrite with caution in patients less than 6 months of age because they may be at higher risk of developing severe methemoglobinemia compared to older children and adults. The presence of fetal hemoglobin, which is oxidized to methemoglobin more easily than adult hemoglobin, and lower methemoglobin reductase levels compared to older children and adults may contribute to risk.
Mortality attributed to sodium nitrite was reported following administration of an adult dose (300 mg IV followed by a second dose of 150 mg) to a 17-month old child. [see Dosage and Administration (2), Warnings and Precautions, (5), Adverse Reactions (6)]
8.5 Geriatric Use
Sodium nitrite and sodium thiosulfate are known to be substantially excreted by the kidney, and the risk of adverse reactions to these drugs may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
Sodium nitrite and sodium thiosulfate are known to be substantially excreted by the kidney, and the risk of toxic reactions to these drugs may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
11. Nithiodote Description
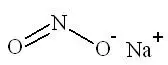
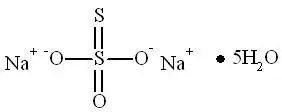
Sodium nitrite, one of the active ingredients in NITHIODOTE has the chemical name nitrous acid sodium salt. The chemical formula is NaNO2 and the molecular weight is 69.0. Sodium thiosulfate, the second active ingredient in NITHIODOTE has the chemical name thiosulfuric acid, disodium salt, pentahydrate. The chemical formula is Na2S2O3∙ 5H2O and the molecular weight is 248.17. The structural formulae are:
Structure of Sodium Nitrite
Structure of Sodium Thiosulfate Pentahydrate
NITHIODOTE is a cyanide antidote which contains one 10 mL glass vial of a 3% solution of sodium nitrite injection and one 50 mL glass vial containing a 25% solution of sodium thiosulfate injection.
Sodium nitrite injection is a sterile aqueous solution and is intended for intravenous injection. Each vial contains 300 mg of sodium nitrite in 10 mL solution (30 mg/mL). Sodium nitrite injection is a clear solution with a pH between 7.0 and 9.0.
Sodium thiosulfate injection is a sterile aqueous solution and is intended for intravenous injection. Each vial contains 12.5 grams of sodium thiosulfate in 50 mL solution (250 mg/mL). Each mL also contains 2.8 mg boric acid and 4.4 mg of potassium chloride. The pH of the solution is adjusted with boric acid and/or sodium hydroxide. Sodium thiosulfate injection is a clear solution with a pH between 7.5 and 9.0.
12. Nithiodote - Clinical Pharmacology
12.1 Mechanism of Action
Cyanide is an extremely toxic poison. In the absence of rapid and adequate treatment, exposure to a high dose of cyanide can result in death within minutes due to the inhibition of cytochrome oxidase resulting in arrest of cellular respiration. Specifically, cyanide binds rapidly with cytochrome a3, a component of the cytochrome c oxidase complex in mitochondria. Inhibition of cytochrome a3 prevents the cell from using oxygen and forces anaerobic metabolism, resulting in lactate production, cellular hypoxia and metabolic acidosis. In massive acute cyanide poisoning, the mechanism of toxicity may involve other enzyme systems as well. Signs and symptoms of acute systemic cyanide poisoning may develop rapidly within minutes, depending on the route and extent of cyanide exposure.
The synergy resulting from treatment of cyanide poisoning with the combination of sodium nitrite and sodium thiosulfate is the result of differences in their primary mechanisms of action as antidotes for cyanide poisoning.
13. Nonclinical Toxicology
16. How is Nithiodote supplied
Each NITHIODOTE carton (NDC 60267-812-00) consists of the following:
- One 10 mL glass vial of sodium nitrite injection 30 mg/mL (containing 300 mg of sodium nitrite);
- One 50 mL glass vial of sodium thiosulfate injection 250 mg/mL (containing 12.5 grams of sodium thiosulfate);
- One package insert.
| NITHIODOTE
sodium nitrite and sodium thiosulfate kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Hope Pharmaceuticals (015227945) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Curia Wisconsin, Inc. | 004670501 | API MANUFACTURE(60267-812) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cangene Biopharma, Inc. | 050783398 | MANUFACTURE(60267-812) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sterling Wisconsin LLC | 961717936 | API MANUFACTURE(60267-812) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Curia New York, Inc. | 124193793 | API MANUFACTURE(60267-812) | |