Drug Detail:Noxafil (Posaconazole (oral/injection) [ poe-sa-kone-a-zole ])
Drug Class: Azole antifungals
Highlights of Prescribing Information
NOXAFIL® (posaconazole) injection, for intravenous use
NOXAFIL® (posaconazole) delayed-release tablets, for oral use
NOXAFIL® (posaconazole) oral suspension
NOXAFIL® PowderMix (posaconazole) for delayed-release oral suspension
Initial U.S. Approval: 2006
Recent Major Changes
| Contraindications (4) | 1/2022 |
| Warnings and Precautions (5) | 1/2022 |
Indications and Usage for Noxafil
Noxafil is an azole antifungal indicated as follows:
- Noxafil injection and Noxafil delayed-release tablets are indicated for the treatment of invasive aspergillosis in adults and pediatric patients 13 years of age and older. (1.1)
-
Noxafil is indicated for the prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy as follows: (1.2)
- Noxafil injection: adults and pediatric patients 2 years of age and older
- Noxafil delayed-release tablets: adults and pediatric patients 2 years of age and older who weigh greater than 40 kg
- Noxafil oral suspension: adults and pediatric patients 13 years of age and older
- Noxafil PowderMix for delayed-release oral suspension: pediatric patients 2 years of age and older who weigh 40 kg or less
- Noxafil oral suspension is indicated for the treatment of oropharyngeal candidiasis (OPC), including OPC refractory (rOPC) to itraconazole and/or fluconazole in adult and pediatric patients aged 13 years and older. (1.3)
Noxafil Dosage and Administration
- Noxafil oral suspension is not substitutable with Noxafil delayed-release tablets or Noxafil PowderMix for delayed-release oral suspension due to the differences in the dosing of each formulation. Therefore, follow the specific dosage recommendations for each of the formulations. (2.1, 2.2, 2.3, 2.8)
- Noxafil injection must be administered through an in-line filter.
- Administer Noxafil injection by intravenous infusion over approximately 90 minutes via a central venous line. (2.1)
- Do NOT administer Noxafil injection as an intravenous bolus injection. (2.1)
- Administer Noxafil delayed-release tablets with or without food. (2.1)
- Administer Noxafil oral suspension with a full meal. (2.1)
- Administer Noxafil PowderMix for delayed-release oral suspension with food. (2.1)
- Administer Noxafil PowderMix for delayed-release oral suspension with the provided notched tip syringes only. (2.1)
| Indication | Dosage Form, Dose, and Duration of Therapy |
|---|---|
| Treatment of invasive Aspergillosis | Noxafil Injection: |
| Loading dose: 300 mg Noxafil injection intravenously twice a day on the first day. |
|
| Maintenance dose: 300 mg Noxafil injection intravenously once a day thereafter. Recommended total duration of therapy is 6 to 12 weeks. (2.2) |
|
| Noxafil Delayed-Release Tablets: | |
| Loading dose: 300 mg (three 100 mg delayed-release tablets) twice a day on the first day. | |
| Maintenance dose: 300 mg (three 100 mg delayed-release tablets) once a day thereafter. Recommended total duration of therapy is 6 to 12 weeks. (2.2) | |
| Switching between the intravenous and delayed-release tablets is acceptable. A loading dose is not required when switching between formulations. (2.2) | |
| Prophylaxis of invasive Aspergillus and Candida infections | Noxafil Injection: |
| Loading dose: 300 mg Noxafil injection intravenously twice a day on the first day. | |
| Maintenance dose: 300 mg Noxafil injection intravenously once a day thereafter. Duration of therapy is based on recovery from neutropenia or immunosuppression. (2.2, 2.3) | |
| Noxafil Delayed-Release Tablets: | |
| Loading dose: 300 mg (three 100 mg delayed-release tablets) twice a day on the first day. | |
| Maintenance dose: 300 mg (three 100 mg delayed-release tablets) once a day, starting on the second day. Duration of therapy is based on recovery from neutropenia or immunosuppression. (2.2, 2.3) | |
| Noxafil Oral Suspension: 200 mg (5 mL) three times a day. Duration of therapy is based on recovery from neutropenia or immunosuppression. (2.2, 2.3) | |
| Oropharyngeal Candidiasis (OPC) | Noxafil Oral Suspension: |
| Loading dose: 100 mg (2.5 mL) twice a day on the first day. | |
| Maintenance dose: 100 mg (2.5 mL) once a day for 13 days. (2.2, 2.3) | |
| OPC Refractory (rOPC) to Itraconazole and/or Fluconazole | Noxafil Oral Suspension: 400 mg (10 mL) twice a day. Duration of therapy is based on the severity of the patient's underlying disease and clinical response. (2.2, 2.3) |
- For pediatric patients, see the Full Prescribing Information for dosing recommendations for Noxafil injection, Noxafil delayed-release tablets, Noxafil oral suspension, and Noxafil PowderMix for delayed-release oral suspension based on the age and indication associated with the dosage form. (1.1, 1.2, 1.3, 2.1, 2.3)
Dosage Forms and Strengths
- Noxafil injection: 300 mg per vial (18 mg per mL) in a single dose vial (3)
- Noxafil delayed-release tablet: 100 mg (3)
- Noxafil oral suspension: 40 mg per mL (3)
- Noxafil PowderMix for delayed-release oral suspension: 300 mg (3)
Contraindications
- Known hypersensitivity to posaconazole or other azole antifungal agents. (4.1)
- Coadministration of Noxafil with the following drugs is contraindicated; Noxafil increases concentrations and toxicities of:
- Sirolimus (4.2, 5.1, 7.1)
- CYP3A4 substrates (pimozide, quinidine): can result in QTc interval prolongation and cases of torsades de pointes (TdP) (4.3, 5.2, 7.2)
- HMG-CoA Reductase Inhibitors Primarily Metabolized through CYP3A4 (4.4, 7.3)
- Ergot alkaloids (4.5, 7.4)
- Venetoclax: in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) at initiation and during the ramp up phase (4.6, 5.10, 7.16)
- Noxafil PowderMix for delayed-release oral suspension is contraindicated in patients with known or suspected Hereditary Fructose Intolerance (HFI). (4.7, 5.8, 8.4)
Warnings and Precautions
- Calcineurin-Inhibitor Toxicity: Noxafil increases concentrations of cyclosporine or tacrolimus; reduce dose of cyclosporine and tacrolimus and monitor concentrations frequently. (5.1)
- Arrhythmias and QTc Prolongation: Noxafil has been shown to prolong the QTc interval and cause cases of TdP. Administer with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs known to prolong QTc interval and metabolized through CYP3A4. (5.2)
- Electrolyte Disturbances: Monitor and correct, especially those involving potassium (K+), magnesium (Mg++), and calcium (Ca++), before and during Noxafil therapy. (5.3)
- Hepatic Toxicity: Elevations in liver tests may occur. Discontinuation should be considered in patients who develop abnormal liver tests or monitor liver tests during treatment. (5.4)
- Renal Impairment: Noxafil injection should be avoided in patients with moderate or severe renal impairment (creatinine clearance <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of Noxafil injection. (5.5, 8.6)
- Concomitant Use with Midazolam: Noxafil can prolong hypnotic/sedative effects. Monitor patients and benzodiazepine receptor antagonists should be available. (5.6, 7.5)
- Vincristine Toxicity: Concomitant administration of azole antifungals, including Noxafil, with vincristine has been associated with neurotoxicity and other serious adverse reactions; reserve azole antifungals, including Noxafil, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options. (5.7, 7.10)
- Risk in Patients with Hereditary Fructose Intolerance (HFI): Noxafil PowderMix for delayed-release oral suspension contains sorbitol. Risk of metabolic crisis with life-threatening hypoglycemia, hypophosphatemia, lactic acidosis, and hepatic failure. Obtain history of HFI symptoms in pediatric patients before Noxafil PowderMix for delayed-release oral suspension administration. (5.8, 8.4)
- Breakthrough Fungal Infections: Monitor patients with severe diarrhea or vomiting when receiving Noxafil delayed-release tablets, Noxafil oral suspension, and Noxafil PowderMix for delayed-release oral suspension. (5.9)
- Venetoclax Toxicity: Concomitant administration of Noxafil with venetoclax may increase venetoclax toxicities, including the risk of tumor lysis syndrome, neutropenia, and serious infections; monitor for toxicity and reduce venetoclax dose. (4.6, 5.10, 7.16)
Adverse Reactions/Side Effects
- Adult Patients: Common adverse reactions in studies with Noxafil in adults are diarrhea, nausea, fever, vomiting, headache, coughing, and hypokalemia. (6.1)
- Pediatric Patients: Common adverse reactions (incidence >20% receiving 6 mg/kg Noxafil injection and Noxafil PowderMix for delayed-release oral suspension) in a study in pediatric patients are pyrexia, febrile neutropenia, vomiting, mucosal inflammation, pruritus, hypertension, hypokalemia, and stomatitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Drug Interactions
| Interaction Drug | Interaction |
|---|---|
|
|
| Rifabutin, phenytoin, efavirenz, cimetidine, esomeprazole* | Avoid coadministration unless the benefit outweighs the risks (7.6, 7.7, 7.8, 7.9) |
| Other drugs metabolized by CYP3A4 | Consider dosage adjustment and monitor for adverse effects and toxicity (7.1, 7.10, 7.11) |
| Digoxin | Monitor digoxin plasma concentrations (7.12) |
| Fosamprenavir, metoclopramide* | Monitor for breakthrough fungal infections (7.6, 7.13) |
Use In Specific Populations
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Pediatrics: Safety and effectiveness in patients younger than 2 years of age have not been established. (8.4)
- Severe Renal Impairment: Monitor closely for breakthrough fungal infections. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2022
Full Prescribing Information
1. Indications and Usage for Noxafil
1.1 Treatment of Invasive Aspergillosis
Noxafil® injection and Noxafil delayed-release tablets are indicated for the treatment of invasive aspergillosis in adults and pediatric patients 13 years of age and older.
1.2 Prophylaxis of Invasive Aspergillus and Candida Infections
Noxafil is indicated for the prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy [see Clinical Studies (14.1)] as follows:
- Noxafil injection: adults and pediatric patients 2 years of age and older
- Noxafil delayed-release tablets: adults and pediatric patients 2 years of age and older who weigh greater than 40 kg
- Noxafil oral suspension: adults and pediatric patients 13 years of age and older
- Noxafil PowderMix for delayed-release oral suspension: pediatric patients 2 years of age and older who weigh 40 kg or less
1.3 Treatment of Oropharyngeal Candidiasis Including Oropharyngeal Candidiasis Refractory to Itraconazole and/or Fluconazole
Noxafil oral suspension is indicated for the treatment of oropharyngeal candidiasis, including oropharyngeal candidiasis refractory to itraconazole and/or fluconazole in adults and pediatric patients 13 years of age and older.
2. Noxafil Dosage and Administration
2.1 Important Administration Instructions
Non-substitutable
Noxafil oral suspension is not substitutable with Noxafil delayed-release tablets or Noxafil PowderMix for delayed-release oral suspension due to the differences in the dosing of each formulation. Therefore, follow the specific dosage recommendations for each of the formulations [see Dosage and Administration (2.2, 2.3, 2.8)].
Noxafil injection
- Administer via a central venous line, including a central venous catheter or peripherally inserted central catheter (PICC), by slow intravenous infusion over approximately 90 minutes [see Dosage and Administration (2.4)].
- If a central venous catheter is not available, Noxafil injection may be administered through a peripheral venous catheter by slow intravenous infusion over 30 minutes only as a single dose in advance of central venous line placement or to bridge the period during which a central venous line is replaced or is in use for other intravenous treatment.
- When multiple dosing is required, the infusion should be done via a central venous line.
- Do NOT administer Noxafil injection as an intravenous bolus injection.
Noxafil delayed-release tablets
- Swallow tablets whole. Do not divide, crush, or chew.
- Administer with or without food [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
- For patients who cannot eat a full meal, Noxafil delayed-release tablets should be used instead of Noxafil oral suspension for the prophylaxis indication. Noxafil delayed-release tablets generally provide higher plasma drug exposures than Noxafil oral suspension under both fed and fasted conditions [see Dosage and Administration (2.6)].
Noxafil oral suspension
- Administer with a full meal or with a liquid nutritional supplement or an acidic carbonated beverage (e.g., ginger ale) in patients who cannot eat a full meal [see Dosage and Administration (2.6)].
- Co-administration of drugs that can decrease the plasma concentrations of posaconazole should generally be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections [see Drug Interactions (7.6, 7.7, 7.8, 7.9, 7.13)].
Noxafil PowderMix for delayed-release oral suspension
- Administer with food [see Clinical Pharmacology (12.3)].
- Administration with alcohol is not recommended [see Drug Interactions (7.15)].
- To ensure delivery of the correct dose, ONLY the provided notched tip syringes must be used for preparation and administration. The design of the notched tip syringe prevents aggregation of the suspension during preparation and administration [see Dosage and Administration (2.8)].
2.2 Dosing Regimen in Adult Patients
| Indication | Dose and Frequency | Duration of Therapy |
|---|---|---|
| Treatment of invasive Aspergillosis |
Noxafil Injection:
Loading dose:
Maintenance dose: Noxafil Delayed-Release Tablets: Loading dose: 300 mg (three 100 mg delayed-release tablets) twice a day on the first day.
Maintenance dose: 300 mg (three 100 mg delayed-release tablets) once a day, starting on the second day. Switching between the intravenous and delayed-release tablets is acceptable. A loading dose is not required when switching between formulations. |
Loading dose:
Maintenance dose:
|
| Prophylaxis of invasive Aspergillus and Candida infections | Noxafil Injection:
Loading dose: 300 mg Noxafil injection intravenously twice a day on the first day. Maintenance dose: 300 mg Noxafil injection intravenously once a day thereafter. Noxafil Delayed-Release Tablets: Loading dose: 300 mg (three 100 mg delayed-release tablets) twice a day on the first day. Maintenance dose: 300 mg (three 100 mg delayed-release tablets) once a day, starting on the second day. Noxafil Oral Suspension: 200 mg (5 mL) three times a day. | Loading dose: 1 day Maintenance dose: Duration of therapy is based on recovery from neutropenia or immunosuppression. |
| Oropharyngeal Candidiasis (OPC) | Noxafil Oral Suspension:
Loading dose: 100 mg (2.5 mL) twice a day on the first day. Maintenance dose: 100 mg (2.5 mL) once a day thereafter. | Loading dose: 1 day Maintenance dose: 13 days |
| OPC Refractory (rOPC) to Itraconazole and/or Fluconazole | Noxafil Oral Suspension: 400 mg (10 mL) twice a day. | Duration of therapy is based on the severity of the patient’s underlying disease and clinical response. |
2.3 Dosing Regimen in Pediatric Patients (ages 2 to less than 18 years of age)
The recommended dosing regimen of Noxafil for pediatric patients 2 to less than 18 years of age is shown in Tables 2, 3, and 4 [see Dosage and Administration (2.5, 2.6, 2.8) and Clinical Pharmacology (12.3)].
Noxafil PowderMix for delayed-release oral suspension is not recommended for use in patients who weigh greater than 40 kg because the recommended dosage cannot be achieved with this formulation.
| Recommended Pediatric Dosage and Formulation | ||||
|---|---|---|---|---|
| Indication | Weight/Age | Delayed-Release Tablet | Injection | Duration of therapy |
| Prophylaxis of invasive Aspergillus and Candida infections | Less than or equal to 40 kg (2 to less than 18 years of age) | Not Applicable | Loading dose:
6 mg/kg up to a maximum of 300 mg twice daily on the first day | Duration of therapy is based on recovery from neutropenia or immunosuppression. |
| Greater than 40 kg (2 to less than 18 years of age) | Loading dose:
300 mg twice daily on the first day Maintenance dose: 300 mg once daily |
Maintenance dose: 6 mg/kg up to a maximum of 300 mg once daily | ||
| Treatment of invasive Aspergillosis | 13 to less than 18 years of age regardless of weight. | Loading dose:
300 mg (three 100 mg delayed-release tablets) twice a day on the first day. | Loading dose:
300 mg Noxafil injection intravenously twice a day on the first day. | Loading dose:
1 day |
|
Maintenance dose: 300 mg (three 100 mg delayed-release tablets) once a day, starting on the second day. |
Maintenance dose: 300 mg Noxafil injection intravenously once a day, starting on the second day. | Maintenance dose:
Recommended total duration of therapy is 6 to 12 weeks. |
||
|
Switching between the intravenous and delayed-release tablets is acceptable. A loading dose is not required when switching between formulations. |
Switching between the intravenous and delayed-release tablets is acceptable. A loading dose is not required when switching between formulations. | |||
| Indication | Loading Dose (volume) and frequency | Maintenance Dose (volume) and frequency | Duration of therapy |
|---|---|---|---|
| Prophylaxis of invasive Aspergillus and Candida infections | 200 mg (5 mL) three times a day | 200 mg (5 mL) three times a day | Duration of therapy is based on recovery from neutropenia or immunosuppression. |
| Oropharyngeal Candidiasis (OPC) | 100 mg (2.5 mL) twice daily on the first day | 100 mg (2.5 mL) once daily | 13 days |
| OPC Refractory (rOPC) to Itraconazole and/or Fluconazole | 400 mg (10 mL) twice daily | 400 mg (10 mL) twice daily | Duration of therapy is based on the severity of the patient’s underlying disease and clinical response. |
| Indication | Weight (kg) | Loading Dose (volume) | Maintenance Dose (volume) |
|---|---|---|---|
| Prophylaxis of invasive Aspergillus and Candida infections | 10 to less than 12 | 90 mg (3 mL) twice daily on the first day | 90 mg (3 mL) once daily |
| 12 to less than 17 | 120 mg (4 mL) twice daily on the first day | 120 mg (4 mL) once daily | |
| 17 to less than 21 | 150 mg (5 mL) twice daily on the first day | 150 mg (5 mL) once daily | |
| 21 to less than 26 | 180 mg (6 mL) twice daily on the first day | 180 mg (6 mL) once daily | |
| 26 to less than 36 | 210 mg (7 mL) twice daily on the first day | 210 mg (7 mL) once daily | |
| 36 to 40 | 240 mg (8 mL) twice daily on the first day | 240 mg (8 mL) once daily |
2.4 Preparation, Intravenous Line Compatibility, and Administration of Noxafil Injection
Preparation:
- Equilibrate the refrigerated vial of Noxafil (posaconazole) injection to room temperature.
- To prepare the required dose, aseptically transfer one vial (16.7 mL) of Noxafil injection (containing 300 mg of posaconazole in solution) to an intravenous bag (or bottle) of a compatible admixture diluent (as described in Table 5), to achieve a final concentration of posaconazole that is between 1 mg/mL and 2 mg/mL. Use of other diluents is not recommended because they may result in particulate formation.
- Noxafil injection is a single-dose sterile solution without preservatives. Discard any unused portion from the vial.
- Once admixed, the diluted solution of Noxafil in the intravenous bag (or bottle) should be used immediately. If not used immediately, the solution can be stored up to 24 hours refrigerated 2 to 8°C (36 to 46°F). Discard any unused portion.
- Parenteral drug products should be inspected visually for particulate matter prior to administration, whenever solution and container permit. Once admixed, the solution of Noxafil ranges from colorless to yellow. Variations of color within this range do not affect the quality of the product.
Intravenous Line Compatibility:
A study was conducted to evaluate physical compatibility of Noxafil injection with injectable drug products and commonly used intravenous diluents during simulated Y-site infusion. Compatibility was determined through visual observations, measurement of particulate matter and turbidity. Compatible diluents and drug products are listed in Tables 5 and 6 below. Any diluents or drug products not listed in the tables below should not be co-administered through the same intravenous line (or cannula).
- Noxafil injection can be infused at the same time through the same intravenous line (or cannula) with the following compatible diluents:
| 0.45% sodium chloride |
| 0.9% sodium chloride |
| 5% dextrose in water |
| 5% dextrose and 0.45% sodium chloride |
| 5% dextrose and 0.9% sodium chloride |
| 5% dextrose and 20 mEq potassium chloride |
- Noxafil injection can be infused at the same time through the same intravenous line (or cannula) with the following drug products prepared in 5% dextrose in water or sodium chloride 0.9%. Co-administration of drug products prepared in other diluents may result in particulate formation.
| Amikacin sulfate |
| Caspofungin |
| Ciprofloxacin |
| Daptomycin |
| Dobutamine hydrochloride |
| Famotidine |
| Filgrastim |
| Gentamicin sulfate |
| Hydromorphone hydrochloride |
| Levofloxacin |
| Lorazepam |
| Meropenem |
| Micafungin |
| Morphine sulfate |
| Norepinephrine bitartrate |
| Potassium chloride |
| Vancomycin hydrochloride |
Incompatible Diluents:
Noxafil injection must not be diluted with the following diluents:
Lactated Ringer's solution
5% dextrose with Lactated Ringer's solution
4.2% sodium bicarbonate
Administration:
- Noxafil injection must be administered through a 0.22-micron polyethersulfone (PES) or polyvinylidene difluoride (PVDF) filter.
- Administer via a central venous line, including a central venous catheter or PICC by slow infusion over approximately 90 minutes. Noxafil injection is not for bolus administration.
- If a central venous catheter is not available, Noxafil injection may be administered through a peripheral venous catheter only as a single dose in advance of central venous line placement or to bridge the period during which a central venous line is replaced or is in use for other treatment.
- When multiple dosing is required, the infusion should be done via a central venous line. When administered through a peripheral venous catheter, the infusion should be administered over approximately 30 minutes. Note: In clinical trials, multiple peripheral infusions given through the same vein resulted in infusion site reactions [see Adverse Reactions (6.1)].
2.5 Administration Instructions for Noxafil Delayed-Release Tablets
- Swallow tablets whole. Do not divide, crush, or chew.
- Administer Noxafil delayed-release tablets with or without food [see Clinical Pharmacology (12.3)].
2.6 Administration Instructions for Noxafil Oral Suspension
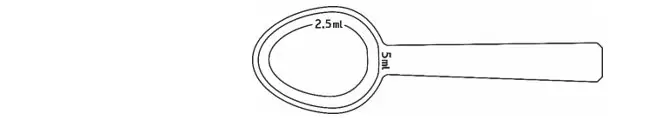
- Shake Noxafil oral suspension well before use. Administer with measured dosing spoon (see Figure 1) provided.
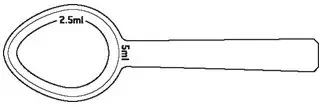 |
| Figure 1: A measured dosing spoon is provided, marked for doses of 2.5 mL and 5 mL. |
- Rinse the spoon with water after each administration and before storage.
- Administer each dose of Noxafil oral suspension during or immediately (i.e., within 20 minutes) following a full meal [see Clinical Pharmacology (12.3)].
- For patients who cannot eat a full meal, Noxafil delayed-release tablets should be used instead of Noxafil oral suspension for the prophylaxis indication. Noxafil delayed-release tablets provide higher plasma drug exposures than Noxafil oral suspension under fasted conditions [see Dosage and Administration (2.1)].
- In patients who cannot eat a full meal and for whom Noxafil delayed-release tablets or Noxafil injection are not options, administer each dose of Noxafil oral suspension with a liquid nutritional supplement or an acidic carbonated beverage (e.g., ginger ale).
- For patients who cannot eat a full meal or tolerate an oral nutritional supplement or an acidic carbonated beverage and who do not have the option of taking Noxafil delayed-release tablets or Noxafil injection, an alternative antifungal therapy should be considered or patients should be monitored closely for breakthrough fungal infections.
2.7 Non-substitutability between Noxafil Oral Suspension and Other Formulations
Noxafil oral suspension is not substitutable with Noxafil delayed-release tablets or Noxafil PowderMix for delayed-release oral suspension due to the differences in the dosing of each formulation. Therefore, follow the specific dosage recommendations for each of the formulations [see Dosage and Administration (2.2, 2.3)].
2.8 Preparation and Administration Instructions for Noxafil PowderMix for Delayed-Release Oral Suspension (pediatric patients ages 2 to less than 18 years of age)
For details on preparation and administration of Noxafil PowderMix for delayed-release oral suspension, see Instructions for Use.
Preparation Instructions
- Do not open packet in the Noxafil PowderMix kit until ready to prepare the medicine.
-
Remove cap from the mixing liquid and push the bottle adapter into the neck of the bottle. Once in place, the bottle adapter stays in the bottle. Remove 9 mL of mixing liquid using the provided BLUE syringe. Put the cap back on the bottle.
ONLY the mixing liquid in the kit should be used to prepare Noxafil PowderMix for delayed-release oral suspension. - Using the provided mixing cup, combine 9 mL of mixing liquid and the entire contents of one packet in the Noxafil PowderMix kit and mix. Each single-use packet in the Noxafil PowderMix kit contains 300 mg of posaconazole to be suspended in 9 mL of mixing liquid giving a final concentration of approximately 30 mg per mL.
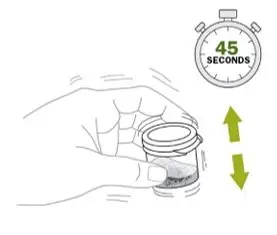
- Shake the mixing cup vigorously for 45 seconds to mix the powder and mixing liquid from the Noxafil PowderMix kit. Check to make sure the powder is mixed. The mixture should look cloudy and free of clumps.
- The reconstituted suspension must be used within 1 hour. Discard unused portion of the prepared drug product.
Administration Instructions
- Administer Noxafil PowderMix for delayed-release oral suspension with food.
-
Choose the correct syringe based on the prescribed dose:
- Use 3 mL (GREEN) syringe if dose is 3 mL or less.
- Use 10 mL (BLUE) syringe if dose is more than 3 mL.
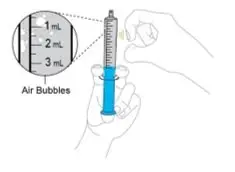
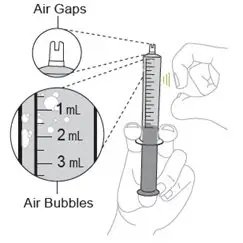
- Measure the prescribed dose volume with the notched tip syringe provided with the kit and administer the dose orally. Administer the dose orally within 1 hour of mixing.
- Not all of the Noxafil PowderMix in the mixing cup will be used. There will be some left over in the mixing cup.
- The maximum dose that can be accurately withdrawn from the mixing cup after reconstitution is 240 mg (8 mL).
- To ensure delivery of the correct dose, ONLY the provided notched tip syringes must be used for preparation and administration. The design of the notched tip syringe prevents aggregation of the suspension during preparation and administration.
- Discard any remaining suspension. The mixing cup may be hand washed and reused. Alternatively, the mixing cup may be discarded, and a similar mixing cup with a lid may be used for subsequent doses.
- The notched tip syringes may also be hand washed and reused. For additional supply, a separate box of notched tip syringes is provided with the Noxafil PowderMix kit.
2.9 Dosage Adjustments in Patients with Renal Impairment
The pharmacokinetics of Noxafil oral suspension and Noxafil delayed-release tablets are not significantly affected by renal impairment. Therefore, no adjustment is necessary for oral dosing in patients with mild to severe renal impairment.
- Noxafil injection should be avoided in patients with moderate or severe renal impairment (eGFR <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of Noxafil injection.
- In patients with moderate or severe renal impairment (estimated glomerular filtration rate (eGFR) <50 mL/min), receiving the Noxafil injection, accumulation of the intravenous vehicle, Betadex Sulfobutyl Ether Sodium (SBECD), is expected to occur. Serum creatinine levels should be closely monitored in these patients, and, if increases occur, consideration should be given to changing to oral Noxafil therapy.
3. Dosage Forms and Strengths
Noxafil injection
Noxafil injection (300 mg per vial) is available as a clear, colorless to yellow sterile liquid in a single-dose vial.
Noxafil Delayed-Release Tablets
Noxafil delayed-release tablets are available as yellow, coated, oblong tablets, debossed with "100" on one side containing 100 mg of posaconazole.
Noxafil Oral Suspension
Noxafil oral suspension is available as a white, cherry-flavored suspension in 4-ounce (123 mL) amber glass bottles with child-resistant closures containing 105 mL of suspension (40 mg of posaconazole per mL).
Noxafil PowderMix for Delayed-Release Oral Suspension
Each Noxafil PowderMix kit contains posaconazole, 300 mg, as an off-white to yellowish powder for delayed-release oral suspension and a mixing liquid.
4. Contraindications
4.1 Hypersensitivity
Noxafil is contraindicated in persons with known hypersensitivity to posaconazole or other azole antifungal agents.
4.2 Use with Sirolimus
Noxafil is contraindicated with sirolimus. Concomitant administration of Noxafil with sirolimus increases the sirolimus blood concentrations by approximately 9-fold and can result in sirolimus toxicity [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
4.3 QT Prolongation with Concomitant Use with CYP3A4 Substrates
Noxafil is contraindicated with CYP3A4 substrates that prolong the QT interval. Concomitant administration of Noxafil with the CYP3A4 substrates, pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2)].
4.4 HMG-CoA Reductase Inhibitors Primarily Metabolized Through CYP3A4
Coadministration with the HMG-CoA reductase inhibitors that are primarily metabolized through CYP3A4 (e.g., atorvastatin, lovastatin, and simvastatin) is contraindicated since increased plasma concentration of these drugs can lead to rhabdomyolysis [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)].
4.5 Use with Ergot Alkaloids
Noxafil may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism [see Drug Interactions (7.4)].
4.6 Use with Venetoclax
Coadministration of Noxafil with venetoclax at initiation and during the ramp-up phase is contraindicated in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) due to the potential for increased risk of tumor lysis syndrome [see Warnings and Precautions (5.10) and Drug Interactions (7.16)].
4.7 Use of Noxafil PowderMix for Delayed-Release Oral Suspension in Patients with Hereditary Fructose Intolerance
Noxafil PowderMix for delayed-release oral suspension is contraindicated in patients with known or suspected hereditary fructose intolerance (HFI) [see Warnings and Precautions (5.8) and Use in Specific Populations (8.4)].
5. Warnings and Precautions
5.1 Calcineurin-Inhibitor Toxicity
Concomitant administration of Noxafil with cyclosporine or tacrolimus increases the whole blood trough concentrations of these calcineurin-inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Nephrotoxicity and leukoencephalopathy (including deaths) have been reported in clinical efficacy studies in patients with elevated cyclosporine or tacrolimus concentrations. Frequent monitoring of tacrolimus or cyclosporine whole blood trough concentrations should be performed during and at discontinuation of Noxafil treatment and the tacrolimus or cyclosporine dose adjusted accordingly.
5.2 Arrhythmias and QT Prolongation
Some azoles, including Noxafil, have been associated with prolongation of the QT interval on the electrocardiogram. In addition, cases of torsades de pointes have been reported in patients taking Noxafil.
Results from a multiple time-matched ECG analysis in healthy volunteers did not show any increase in the mean of the QTc interval. Multiple, time-matched ECGs collected over a 12-hour period were recorded at baseline and steady-state from 173 healthy male and female volunteers (18-85 years of age) administered Noxafil oral suspension 400 mg twice daily with a high-fat meal. In this pooled analysis, the mean QTc (Fridericia) interval change from baseline was –5 msec following administration of the recommended clinical dose. A decrease in the QTc(F) interval (–3 msec) was also observed in a small number of subjects (n=16) administered placebo. The placebo-adjusted mean maximum QTc(F) interval change from baseline was <0 msec (–8 msec). No healthy subject administered Noxafil had a QTc(F) interval ≥500 msec or an increase ≥60 msec in their QTc(F) interval from baseline.
Noxafil should be administered with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs that are known to prolong the QTc interval and are metabolized through CYP3A4 [see Contraindications (4.3) and Drug Interactions (7.2)].
5.3 Electrolyte Disturbances
Electrolyte disturbances, especially those involving potassium, magnesium or calcium levels, should be monitored and corrected as necessary before and during Noxafil therapy.
5.4 Hepatic Toxicity
Hepatic reactions (e.g., mild to moderate elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin, and/or clinical hepatitis) have been reported in clinical trials. The elevations in liver tests were generally reversible on discontinuation of therapy, and in some instances these tests normalized without drug interruption. Cases of more severe hepatic reactions including cholestasis or hepatic failure including deaths have been reported in patients with serious underlying medical conditions (e.g., hematologic malignancy) during treatment with Noxafil. These severe hepatic reactions were seen primarily in subjects receiving the Noxafil oral suspension 800 mg daily (400 mg twice daily or 200 mg four times a day) in clinical trials.
Liver tests should be evaluated at the start of and during the course of Noxafil therapy. Patients who develop abnormal liver tests during Noxafil therapy should be monitored for the development of more severe hepatic injury. Patient management should include laboratory evaluation of hepatic function (particularly liver tests and bilirubin). Discontinuation of Noxafil must be considered if clinical signs and symptoms consistent with liver disease develop that may be attributable to Noxafil.
5.5 Renal Impairment
Due to the variability in exposure with Noxafil delayed-release tablets, Noxafil oral suspension, and Noxafil PowderMix for delayed-release oral suspension, patients with severe renal impairment should be monitored closely for breakthrough fungal infections [see Dosage and Administration (2.9) and Use in Specific Populations (8.6)].
Noxafil injection should be avoided in patients with moderate or severe renal impairment (eGFR <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of Noxafil injection. In patients with moderate or severe renal impairment (eGFR <50 mL/min), receiving the Noxafil injection, accumulation of the intravenous vehicle, SBECD, is expected to occur. Serum creatinine levels should be closely monitored in these patients, and, if increases occur, consideration should be given to changing to oral Noxafil therapy [see Dosage and Administration (2.9) and Use in Specific Populations (8.6)].
5.6 Midazolam Toxicity
Concomitant administration of Noxafil with midazolam increases the midazolam plasma concentrations by approximately 5-fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Patients must be monitored closely for adverse effects associated with high plasma concentrations of midazolam and benzodiazepine receptor antagonists must be available to reverse these effects [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)].
5.7 Vincristine Toxicity
Concomitant administration of azole antifungals, including Noxafil, with vincristine has been associated with neurotoxicity and other serious adverse reactions, including seizures, peripheral neuropathy, syndrome of inappropriate antidiuretic hormone secretion, and paralytic ileus. Reserve azole antifungals, including Noxafil, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options [see Drug Interactions (7.10)].
5.8 Risk in Patients with Hereditary Fructose Intolerance (HFI)
Noxafil PowderMix for delayed-release oral suspension contains sorbitol, an inactive ingredient, and may precipitate a metabolic crisis that may include, but is not limited to life-threatening hypoglycemia, hypophosphatemia, lactic acidosis, and hepatic failure in patients with HFI. Obtain careful history of HFI symptoms (nausea, vomiting, abdominal pain) with sorbitol/fructose/sucrose exposure prior to Noxafil PowderMix for delayed-release oral suspension administration because a diagnosis of HFI may not yet be established in pediatric patients [see Contraindications (4), Use in Specific Populations (8.4)].
5.9 Breakthrough Fungal Infections
Patients who have severe diarrhea or vomiting should be monitored closely for breakthrough fungal infections when receiving Noxafil delayed-release tablets, Noxafil oral suspension, or Noxafil PowderMix for delayed-release oral suspension.
5.10 Venetoclax Toxicity
Concomitant administration of Noxafil, a strong CYP3A4 inhibitor, with venetoclax may increase venetoclax toxicities, including the risk of tumor lysis syndrome (TLS), neutropenia, and serious infections. In patients with CLL/SLL, administration of Noxafil during initiation and the ramp-up phase of venetoclax is contraindicated [see Contraindications (4.6)]. Refer to the venetoclax labeling for safety monitoring and dose reduction in the steady daily dosing phase in CLL/SLL patients.
For patients with acute myeloid leukemia (AML), dose reduction and safety monitoring are recommended across all dosing phases when coadministering Noxafil with venetoclax [see Drug Interactions (7.16)]. Refer to the venetoclax prescribing information for dosing instructions.
6. Adverse Reactions/Side Effects
The following serious and otherwise important adverse reactions are discussed in detail in another section of the labeling:
- Hypersensitivity [see Contraindications (4.1)]
- Arrhythmias and QT Prolongation [see Warnings and Precautions (5.2)]
- Hepatic Toxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of Noxafil cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trial Experience in Adults
Clinical Trial Experience with Noxafil Injection and Noxafil Delayed-Release Tablets for the Treatment of Invasive Aspergillosis
The safety of Noxafil injection and Noxafil delayed-release tablet was assessed in a randomized, double-blind, active-controlled clinical study of Noxafil injection and Noxafil delayed-release tablets versus voriconazole for treatment of invasive aspergillosis (Aspergillosis Treatment Study). A total of 575 (288 in Noxafil arm, 287 in voriconazole arm) adult and pediatric patients 13 years of age and older with proven, probable or possible invasive aspergillosis were included. The median duration of treatment was 67 days for Noxafil injection or Noxafil delayed-release tablet and 64 days for voriconazole, with 55% to 60% of subjects starting treatment with the IV formulation of either drug. The median duration of the first instance of IV treatment (before switching to oral treatment or discontinuing or completing study treatment) was 9 days for both groups. Table 7 presents adverse reactions reported at an incidence of ≥10% in either one of the groups in Aspergillosis Treatment Study.
Adverse reactions leading to treatment discontinuation were reported for 33.9% of subjects. The most commonly reported adverse reactions (>2% of subjects) leading to treatment discontinuation were septic shock, respiratory failure, and bronchopulmonary aspergillosis in the Noxafil arm, and septic shock and acute myeloid leukemia in the voriconazole arm.
| System Organ Class | Noxafil injection or tablet (N = 288), n (%) | Voriconazole injection or oral (N = 287), n (%) |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Anemia | 25 (8.7) | 29 (10.1) |
| Febrile neutropenia | 42 (14.6) | 38 (13.2) |
| Gastrointestinal disorders | ||
| Abdominal pain | 29 (10.1) | 24 (8.4) |
| Constipation | 32 (11.1) | 23 (8.0) |
| Diarrhea | 52 (18.1) | 52 (18.1) |
| Nausea | 65 (22.6) | 51 (17.8) |
| Vomiting | 52 (18.1) | 39 (13.6) |
| General disorders and administration site conditions | ||
| Edema peripheral | 32 (11.1) | 24 (8.4) |
| Pyrexia | 81 (28.1) | 72 (25.1) |
| Infections and infestations | ||
| Pneumonia | 36 (12.5) | 26 (9.1) |
| Investigations | ||
| Alanine aminotransferase increased | 42 (14.6) | 37 (12.9) |
| Aspartate aminotransferase increased | 38 (13.2) | 36 (12.5) |
| Blood alkaline phosphatase increased | 21 (7.3) | 29 (10.1) |
| Metabolism and nutrition disorders | ||
| Hypokalemia | 82 (28.5) | 49 (17.1) |
| Hypomagnesemia | 29 (10.1) | 18 (6.3) |
| Nervous system disorders | ||
| Headache | 35 (12.2) | 25 (8.7) |
| Respiratory, thoracic and mediastinal disorders | ||
| Cough | 30 (10.4) | 24 (8.4) |
| Epistaxis | 32 (11.1) | 17 (5.9) |
The most frequently reported adverse reactions in the Noxafil-treated group were pyrexia (28%), hypokalemia (28%), and nausea (23%).
Clinical Trial Experience with Noxafil Injection for Prophylaxis
Multiple doses of Noxafil injection administered via a peripheral venous catheter were associated with thrombophlebitis (60% incidence). Therefore, in subsequent studies, Noxafil injection was administered via central venous catheter.
The safety of Noxafil injection has been assessed in 268 patients in a clinical trial. Patients were enrolled in a non-comparative pharmacokinetic and safety trial of Noxafil injection when given as antifungal prophylaxis (Noxafil Injection Study). Patients were immunocompromised with underlying conditions including hematological malignancy, neutropenia post-chemotherapy, GVHD, and post HSCT. This patient population was 55% male, had a mean age of 51 years (range 18-82 years, 19% of patients were ≥65 years of age), and were 95% white and 8% Hispanic. Ten patients received a single dose of 200 mg Noxafil injection, 21 patients received 200 mg daily dose for a median of 14 days, and 237 patients received 300 mg daily dose for a median of 9 days.
Table 8 presents adverse reactions observed in patients treated with Noxafil injection 300 mg daily dose in the Noxafil Injection Study. Each patient received a loading dose, 300 mg twice on Day 1. Following Noxafil intravenous therapy, patients received Noxafil oral suspension to complete 28 days of total Noxafil therapy.
| Body System | Noxafil Injection Treatment Phase n=237 (%)* | Noxafil Injection Treatment Phase or Subsequent Noxafil Oral Suspension Treatment Phase n=237 (%)† |
||
|---|---|---|---|---|
|
||||
| Subjects Reporting any Adverse Reaction | 220 | (93) | 235 | (99) |
| Blood and Lymphatic System Disorder | ||||
| Anemia | 16 | (7) | 23 | (10) |
| Thrombocytopenia | 17 | (7) | 25 | (11) |
| Gastrointestinal Disorders | ||||
| Abdominal Pain Upper | 15 | (6) | 25 | (11) |
| Abdominal Pain | 30 | (13) | 41 | (17) |
| Constipation | 18 | (8) | 31 | (13) |
| Diarrhea | 75 | (32) | 93 | (39) |
| Nausea | 46 | (19) | 70 | (30) |
| Vomiting | 29 | (12) | 45 | (19) |
| General Disorders and Administration Site Conditions | ||||
| Fatigue | 19 | (8) | 24 | (10) |
| Chills | 28 | (12) | 38 | (16) |
| Edema Peripheral | 28 | (12) | 35 | (15) |
| Pyrexia | 49 | (21) | 73 | (31) |
| Metabolism and Nutrition Disorders | ||||
| Decreased appetite | 23 | (10) | 29 | (12) |
| Hypokalemia | 51 | (22) | 67 | (28) |
| Hypomagnesemia | 25 | (11) | 30 | (13) |
| Nervous System Disorders | ||||
| Headache | 33 | (14) | 49 | (21) |
| Respiratory, Thoracic and Mediastinal Disorders | ||||
| Cough | 21 | (9) | 31 | (13) |
| Dyspnea | 16 | (7) | 24 | (10) |
| Epistaxis | 34 | (14) | 40 | (17) |
| Skin and Subcutaneous Tissue Disorders | ||||
| Petechiae | 20 | (8) | 24 | (10) |
| Rash | 35 | (15) | 56 | (24) |
| Vascular Disorders | ||||
| Hypertension | 20 | (8) | 26 | (11) |
The most frequently reported adverse reactions with an onset during the Noxafil intravenous phase of dosing with 300 mg once daily were diarrhea (32%), hypokalemia (22%), pyrexia (21%), and nausea (19%). These adverse reactions were consistent with those seen in studies with Noxafil oral suspension.
Clinical Trial Experience with Noxafil Delayed-Release Tablets for Prophylaxis
The safety of Noxafil delayed-release tablets has been assessed in 230 patients in clinical trials. Patients were enrolled in a non-comparative pharmacokinetic and safety trial of Noxafil delayed-release tablets when given as antifungal prophylaxis (Noxafil Delayed-Release Tablet Study). Patients were immunocompromised with underlying conditions including hematological malignancy, neutropenia post-chemotherapy, GVHD, and post HSCT. This patient population was 62% male, had a mean age of 51 years (range 19-78 years, 17% of patients were ≥65 years of age), and were 93% white and 16% Hispanic. Posaconazole therapy was given for a median duration of 28 days. Twenty patients received 200 mg daily dose and 210 patients received 300 mg daily dose (following twice daily dosing on Day 1 in each cohort). Table 9 presents adverse reactions observed in patients treated with 300 mg daily dose at an incidence of ≥10% in Noxafil Delayed-Release Tablet Study.
| Body System | Noxafil delayed-release tablet (300 mg) n=210 (%) |
|
|---|---|---|
| Subjects Reporting any Adverse Reaction | 207 | (99) |
| Blood and Lymphatic System Disorder | ||
| Anemia | 22 | (10) |
| Thrombocytopenia | 29 | (14) |
| Gastrointestinal Disorders | ||
| Abdominal Pain | 23 | (11) |
| Constipation | 20 | (10) |
| Diarrhea | 61 | (29) |
| Nausea | 56 | (27) |
| Vomiting | 28 | (13) |
| General Disorders and Administration Site Conditions | ||
| Asthenia | 20 | (10) |
| Chills | 22 | (10) |
| Mucosal Inflammation | 29 | (14) |
| Edema Peripheral | 33 | (16) |
| Pyrexia | 59 | (28) |
| Metabolism and Nutrition Disorders | ||
| Hypokalemia | 46 | (22) |
| Hypomagnesemia | 20 | (10) |
| Nervous System Disorders | ||
| Headache | 30 | (14) |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Cough | 35 | (17) |
| Epistaxis | 30 | (14) |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash | 34 | (16) |
| Vascular Disorders | ||
| Hypertension | 23 | (11) |
The most frequently reported adverse reactions (>25%) with Noxafil delayed-release tablets 300 mg once daily were diarrhea, pyrexia, and nausea.
The most common adverse reaction leading to discontinuation of Noxafil delayed-release tablets 300 mg once daily was nausea (2%).
Clinical Trial Safety Experience with Noxafil Oral Suspension
The safety of Noxafil oral suspension has been assessed in 1844 patients. This includes 605 patients in the active-controlled prophylaxis studies, 557 patients in the active-controlled OPC studies, 239 patients in refractory OPC studies, and 443 patients from other indications. This represents a heterogeneous population, including immunocompromised patients, e.g., patients with hematological malignancy, neutropenia post-chemotherapy, GVHD post HSCT, and HIV infection, as well as non-neutropenic patients. This patient population was 71% male, had a mean age of 42 years (range 8-84 years, 6% of patients were ≥65 years of age and 1% was <18 years of age), and were 64% white, 16% Hispanic, and 36% non-white (including 14% black). Noxafil therapy was given to 171 patients for ≥6 months, with 58 patients receiving Noxafil therapy for ≥12 months. Table 10 presents adverse reactions observed at an incidence of >10% in Noxafil prophylaxis studies. Table 11 presents adverse reactions observed at an incidence of at least 10% in the OPC/rOPC studies.
Prophylaxis of Aspergillus and Candida: In the 2 randomized, comparative prophylaxis studies (Noxafil Oral Suspension Study 1 and 2), the safety of Noxafil oral suspension 200 mg three times a day was compared to fluconazole 400 mg once daily or itraconazole 200 mg twice a day in severely immunocompromised patients.
The most frequently reported adverse reactions (>30%) in the prophylaxis clinical trials were fever, diarrhea, and nausea.
The most common adverse reactions leading to discontinuation of Noxafil in the prophylaxis studies were associated with GI disorders, specifically, nausea (2%), vomiting (2%), and hepatic enzymes increased (2%).
| Body System | Noxafil Oral Suspension n=605 (%) | Fluconazole n=539 (%) | Itraconazole n=58 (%) |
|||
|---|---|---|---|---|---|---|
|
||||||
| Subjects Reporting any Adverse Reaction | 595 | (98) | 531 | (99) | 58 | (100) |
| Body as a Whole - General Disorders | ||||||
| Fever | 274 | (45) | 254 | (47) | 32 | (55) |
| Headache | 171 | (28) | 141 | (26) | 23 | (40) |
| Rigors | 122 | (20) | 87 | (16) | 17 | (29) |
| Fatigue | 101 | (17) | 98 | (18) | 5 | (9) |
| Edema Legs | 93 | (15) | 67 | (12) | 11 | (19) |
| Anorexia | 92 | (15) | 94 | (17) | 16 | (28) |
| Dizziness | 64 | (11) | 56 | (10) | 5 | (9) |
| Edema | 54 | (9) | 68 | (13) | 8 | (14) |
| Weakness | 51 | (8) | 52 | (10) | 2 | (3) |
| Cardiovascular Disorders, General | ||||||
| Hypertension | 106 | (18) | 88 | (16) | 3 | (5) |
| Hypotension | 83 | (14) | 79 | (15) | 10 | (17) |
| Disorders of Blood and Lymphatic System | ||||||
| Anemia | 149 | (25) | 124 | (23) | 16 | (28) |
| Neutropenia | 141 | (23) | 122 | (23) | 23 | (40) |
| Disorders of the Reproductive System and Breast | ||||||
| Vaginal Hemorrhage* | 24 | (10) | 20 | (9) | 3 | (12) |
| Gastrointestinal System Disorders | ||||||
| Diarrhea | 256 | (42) | 212 | (39) | 35 | (60) |
| Nausea | 232 | (38) | 198 | (37) | 30 | (52) |
| Vomiting | 174 | (29) | 173 | (32) | 24 | (41) |
| Abdominal Pain | 161 | (27) | 147 | (27) | 21 | (36) |
| Constipation | 126 | (21) | 94 | (17) | 10 | (17) |
| Dyspepsia | 61 | (10) | 50 | (9) | 6 | (10) |
| Heart Rate and Rhythm Disorders | ||||||
| Tachycardia | 72 | (12) | 75 | (14) | 3 | (5) |
| Infection and Infestations | ||||||
| Pharyngitis | 71 | (12) | 60 | (11) | 12 | (21) |
| Liver and Biliary System Disorders | ||||||
| Bilirubinemia | 59 | (10) | 51 | (9) | 11 | (19) |
| Metabolic and Nutritional Disorders | ||||||
| Hypokalemia | 181 | (30) | 142 | (26) | 30 | (52) |
| Hypomagnesemia | 110 | (18) | 84 | (16) | 11 | (19) |
| Hyperglycemia | 68 | (11) | 76 | (14) | 2 | (3) |
| Hypocalcemia | 56 | (9) | 55 | (10) | 5 | (9) |
| Musculoskeletal System Disorders | ||||||
| Musculoskeletal Pain | 95 | (16) | 82 | (15) | 9 | (16) |
| Arthralgia | 69 | (11) | 67 | (12) | 5 | (9) |
| Back Pain | 63 | (10) | 66 | (12) | 4 | (7) |
| Platelet, Bleeding and Clotting Disorders | ||||||
| Thrombocytopenia | 175 | (29) | 146 | (27) | 20 | (34) |
| Petechiae | 64 | (11) | 54 | (10) | 9 | (16) |
| Psychiatric Disorders | ||||||
| Insomnia | 103 | (17) | 92 | (17) | 11 | (19) |
| Respiratory System Disorders | ||||||
| Coughing | 146 | (24) | 130 | (24) | 14 | (24) |
| Dyspnea | 121 | (20) | 116 | (22) | 15 | (26) |
| Epistaxis | 82 | (14) | 73 | (14) | 12 | (21) |
| Skin and Subcutaneous Tissue Disorders | ||||||
| Rash | 113 | (19) | 96 | (18) | 25 | (43) |
| Pruritus | 69 | (11) | 62 | (12) | 11 | (19) |
HIV Infected Subjects with OPC: In 2 randomized comparative studies in OPC, the safety of Noxafil oral suspension at a dose of less than or equal to 400 mg once daily in 557 HIV-infected patients was compared to the safety of fluconazole in 262 HIV-infected patients at a dose of 100 mg once daily.
An additional 239 HIV-infected patients with refractory OPC received Noxafil oral suspension in 2 non-comparative trials for refractory OPC (rOPC). Of these subjects, 149 received the 800-mg/day dose and the remainder received the less than or equal to 400-mg once daily dose.
In the OPC/rOPC studies, the most common adverse reactions were fever, diarrhea, nausea, headache, vomiting, and coughing.
The most common adverse reactions that led to treatment discontinuation of Noxafil in the Controlled OPC Pool included respiratory impairment (1%) and pneumonia (1%). In the refractory OPC pool, the most common adverse reactions that led to treatment discontinuation of Noxafil were AIDS (7%) and respiratory impairment (3%).
| Body System | Number (%) of Subjects | ||
|---|---|---|---|
| Controlled OPC Pool | Refractory OPC Pool | ||
| Noxafil Oral Suspension | Fluconazole | Noxafil Oral Suspension |
|
| n=557 | n=262 | n=239 | |
| OPC=oropharyngeal candidiasis | |||
|
|||
| Subjects Reporting any Adverse Reaction* | 356 (64) | 175 (67) | 221 (92) |
| Body as a Whole – General Disorders | |||
| Fever | 34 (6) | 22 (8) | 82 (34) |
| Headache | 44 (8) | 23 (9) | 47 (20) |
| Anorexia | 10 (2) | 4 (2) | 46 (19) |
| Fatigue | 18 (3) | 12 (5) | 31 (13) |
| Asthenia | 9 (2) | 5 (2) | 31 (13) |
| Rigors | 2 (<1) | 4 (2) | 29 (12) |
| Pain | 4 (1) | 2 (1) | 27 (11) |
| Disorders of Blood and Lymphatic System | |||
| Neutropenia | 21 (4) | 8 (3) | 39 (16) |
| Anemia | 11 (2) | 5 (2) | 34 (14) |
| Gastrointestinal System Disorders | |||
| Diarrhea | 58 (10) | 34 (13) | 70 (29) |
| Nausea | 48 (9) | 30 (11) | 70 (29) |
| Vomiting | 37 (7) | 18 (7) | 67 (28) |
| Abdominal Pain | 27 (5) | 17 (6) | 43 (18) |
| Infection and Infestations | |||
| Candidiasis, Oral | 3 (1) | 1 (<1) | 28 (12) |
| Herpes Simplex | 16 (3) | 8 (3) | 26 (11) |
| Pneumonia | 17 (3) | 6 (2) | 25 (10) |
| Metabolic and Nutritional Disorders | |||
| Weight Decrease | 4 (1) | 2 (1) | 33 (14) |
| Dehydration | 4 (1) | 7 (3) | 27 (11) |
| Psychiatric Disorders | |||
| Insomnia | 8 (1) | 3 (1) | 39 (16) |
| Respiratory System Disorders | |||
| Coughing | 18 (3) | 11 (4) | 60 (25) |
| Dyspnea | 8 (1) | 8 (3) | 28 (12) |
| Skin and Subcutaneous Tissue Disorders | |||
| Rash | 15 (3) | 10 (4) | 36 (15) |
| Sweating Increased | 13 (2) | 5 (2) | 23 (10) |
Adverse reactions were reported more frequently in the pool of patients with refractory OPC. Among these highly immunocompromised patients with advanced HIV disease, serious adverse reactions (SARs) were reported in 55% (132/239). The most commonly reported SARs were fever (13%) and neutropenia (10%).
Clinical Laboratory Values: In healthy volunteers and patients, elevation of liver test values did not appear to be associated with higher plasma concentrations of posaconazole.
For the prophylaxis studies, the number of patients with changes in liver tests from Common Toxicity Criteria (CTC) Grade 0, 1, or 2 at baseline to Grade 3 or 4 during the study is presented in Table 12.
| Number (%) of Patients with Change*
Noxafil Oral Suspension Study 1 |
||
|---|---|---|
| CTC = Common Toxicity Criteria; AST= Aspartate Aminotransferase; | ||
| ALT= Alanine Aminotransferase. | ||
|
||
| Laboratory Parameter | Noxafil Oral Suspension n=301 | Fluconazole n=299 |
| AST | 11/266 (4) | 13/266 (5) |
| ALT | 47/271 (17) | 39/272 (14) |
| Bilirubin | 24/271 (9) | 20/275 (7) |
| Alkaline Phosphatase | 9/271 (3) | 8/271 (3) |
| Noxafil Oral Suspension Study 2 | ||
| Laboratory Parameter | Noxafil Oral Suspension (n=304) | Fluconazole/Itraconazole (n=298) |
| AST | 9/286 (3) | 5/280 (2) |
| ALT | 18/289 (6) | 13/284 (5) |
| Bilirubin | 20/290 (7) | 25/285 (9) |
| Alkaline Phosphatase | 4/281 (1) | 1/276 (<1) |
The number of patients treated for OPC with clinically significant liver test abnormalities at any time during the studies is provided in Table 13 (liver test abnormalities were present in some of these patients prior to initiation of the study drug).
| Laboratory Test | Controlled | Refractory | |
|---|---|---|---|
| Noxafil Oral Suspension | Fluconazole | Noxafil Oral Suspension | |
| n=557 (%) | n=262 (%) | n=239 (%) | |
| ALT= Alanine Aminotransferase; AST= Aspartate Aminotransferase. | |||
| ALT > 3.0 × ULN | 16/537 (3) | 13/254 (5) | 25/226 (11) |
| AST > 3.0 × ULN | 33/537 (6) | 26/254 (10) | 39/223 (17) |
| Total Bilirubin > 1.5 × ULN | 15/536 (3) | 5/254 (2) | 9/197 (5) |
| Alkaline Phosphatase > 3.0 × ULN | 17/535 (3) | 15/253 (6) | 24/190 (13) |
The number of patients treated for invasive aspergillosis with clinically significant liver test abnormalities at any time during the Aspergillosis Treatment Study is provided in Table 14. Liver test abnormalities present prior to the initiation of study drug included ALT (22%), AST (13%), and bilirubin (13%).
| Number (%) of Patients with Change* | ||
|---|---|---|
| Laboratory Parameter | Noxafil n/N (%) | Voriconazole n/N (%) |
| N=Number of subjects for a given laboratory test with a baseline value of CTC Grade 0, 1, or 2 and at least one post-baseline value. | ||
| CTC = Common Toxicity Criteria; AST= Aspartate Aminotransferase; | ||
| ALT= Alanine Aminotransferase. | ||
|
||
| AST | 22/281 (8) | 21/285 (7) |
| ALT | 29/281(10) | 23/282 (8) |
| Bilirubin | 26/280 (9) | 25/284 (9) |
| Alkaline Phosphatase | 12/282 (4) | 20/284 (7) |
Clinical Trial Experience in Pediatrics
Clinical Trial Experience in Pediatric Patients (2 to less than 18 Years of Age)
The safety of Noxafil injection and Noxafil PowderMix for delayed-release oral suspension for prophylaxis of invasive fungal infections has been assessed in an open label uncontrolled dose-ranging PK and safety study (Noxafil injection/ Noxafil PowderMix for delayed-release oral suspension Pediatric Study 1, NCT02452034); hereinafter referred to as Noxafil Pediatric Study) in 115 immunocompromised pediatric patients 2 to less than 18 years of age with known or expected neutropenia. Noxafil injection and Noxafil PowderMix for delayed-release oral suspension was administered at daily doses of up to 6 mg/kg (twice daily on day 1) in three dose cohorts. All 115 subjects initially received Noxafil injection for at least 7 days, and 63 subjects were transitioned to Noxafil PowderMix for delayed-release oral suspension. The mean overall treatment duration for all treated subjects was 20.6 days with 14.3 days (range: 1 to 28 days) on Noxafil injection and 11.6 days (range: 2 to 18 days) on Noxafil PowderMix for delayed-release oral suspension [see Clinical Pharmacology (12.3)].
Table 15 presents adverse reactions observed in greater than or equal to 10% of pediatric patients treated with Noxafil in the Noxafil Pediatric Study.
Reported adverse reaction profile of Noxafil in pediatric patients was consistent with the safety profile of Noxafil in adults. The most common adverse reactions (occurring in greater than 20% of pediatric patients receiving 6 mg/kg Noxafil injection and Noxafil PowderMix for delayed-release oral suspension daily dose) were pyrexia, febrile neutropenia, vomiting, mucosal inflammation, pruritus, hypertension, hypokalemia, and stomatitis.
| Adverse Reaction | Noxafil Injection and Noxafil PowderMix for Delayed-Release Oral Suspension 6 mg/kg Dose Cohort n=49 (%) | Noxafil Injection and Noxafil PowderMix for Delayed-Release Oral Suspension All Dose Cohorts n=115 (%) |
|---|---|---|
| Pyrexia | 16 (33) | 50 (43) |
| Febrile neutropenia | 15 (31) | 25 (22) |
| Vomiting | 12 (24) | 30 (26) |
| Mucosal inflammation | 11 (22) | 32 (28) |
| Pruritus | 11 (22) | 18 (16) |
| Hypertension | 10 (20) | 20 (17) |
| Hypokalemia | 10 (20) | 16 (14) |
| Stomatitis | 10 (20) | 13 (11) |
| Diarrhea | 9 (18) | 25 (22) |
| Nausea | 9 (18) | 18 (16) |
| Abdominal pain | 8 (16) | 20 (17) |
| Decreased appetite | 7 (14) | 17 (15) |
| Rash | 7 (14) | 18 (16) |
| Alanine aminotransferase increased | 6 (12) | 8 (7) |
| Headache | 6 (12) | 16 (14) |
| Aspartate aminotransferase increased | 5 (10) | 8 (7) |
The number of patients receiving Noxafil in the Noxafil Pediatric Study who had changes in liver tests from Grade 0, 1, or 2 at baseline to Grade 3 or 4 is presented in Table 16.
| Number (%) of Patients with Change*
Pediatric Study 1 |
|
|---|---|
| Laboratory Parameter | Noxafil Injection and Noxafil PowderMix for Delayed- Release Oral Suspension (6 mg/kg daily) n=49 (%) |
| CTC = Common Toxicity Criteria; AST= Aspartate Aminotransferase; ALT= Alanine Aminotransferase |
|
|
|
| AST | 2/49 (4) |
| ALT | 3/49 (6) |
| Bilirubin | 0/48 (0) |
| Alkaline Phosphatase | 0/48 (0) |
7. Drug Interactions
Posaconazole is primarily metabolized via UDP glucuronosyltransferase and is a substrate of p-glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. Coadministration of drugs that can decrease the plasma concentrations of posaconazole should generally be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Posaconazole is also a strong inhibitor of CYP3A4. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole [see Clinical Pharmacology (12.3)].
The following information was derived from data with Noxafil oral suspension or early tablet formulation unless otherwise noted. All drug interactions with Noxafil oral suspension, except for those that affect the absorption of posaconazole (via gastric pH and motility), are considered relevant to Noxafil injection, Noxafil delayed-release tablet, and Noxafil PowderMix for delayed-release oral suspension as well [see Drug Interactions (7.9) and (7.13)].
7.2 CYP3A4 Substrates
Concomitant administration of Noxafil with CYP3A4 substrates such as pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes. Therefore, Noxafil is contraindicated with these drugs [see Contraindications (4.3) and Warnings and Precautions (5.2)].
7.3 HMG-CoA Reductase Inhibitors (Statins) Primarily Metabolized Through CYP3A4
Concomitant administration of Noxafil with simvastatin increases the simvastatin plasma concentrations by approximately 10-fold. Therefore, Noxafil is contraindicated with HMG-CoA reductase inhibitors primarily metabolized through CYP3A4 [see Contraindications (4.4) and Clinical Pharmacology (12.3)].
7.4 Ergot Alkaloids
Most of the ergot alkaloids are substrates of CYP3A4. Noxafil may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism. Therefore, Noxafil is contraindicated with ergot alkaloids [see Contraindications (4.5)].
7.5 Benzodiazepines Metabolized by CYP3A4
Concomitant administration of Noxafil with midazolam increases the midazolam plasma concentrations by approximately 5-fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Concomitant use of Noxafil and other benzodiazepines metabolized by CYP3A4 (e.g., alprazolam, triazolam) could result in increased plasma concentrations of these benzodiazepines. Patients must be monitored closely for adverse effects associated with high plasma concentrations of benzodiazepines metabolized by CYP3A4 and benzodiazepine receptor antagonists must be available to reverse these effects [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
7.7 Rifabutin
Rifabutin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Rifabutin is also metabolized by CYP3A4. Therefore, coadministration of rifabutin with Noxafil increases rifabutin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of Noxafil and rifabutin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring for breakthrough fungal infections as well as frequent monitoring of full blood counts and adverse reactions due to increased rifabutin plasma concentrations (e.g., uveitis, leukopenia) are recommended.
7.8 Phenytoin
Phenytoin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Phenytoin is also metabolized by CYP3A4. Therefore, coadministration of phenytoin with Noxafil increases phenytoin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of Noxafil and phenytoin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring for breakthrough fungal infections is recommended and frequent monitoring of phenytoin concentrations should be performed while coadministered with Noxafil and dose reduction of phenytoin should be considered.
7.10 Vinca Alkaloids
Most of the vinca alkaloids (e.g., vincristine and vinblastine) are substrates of CYP3A4. Concomitant administration of azole antifungals, including Noxafil, with vincristine has been associated with serious adverse reactions [see Warnings and Precautions (5.7)]. Noxafil may increase the plasma concentrations of vinca alkaloids which may lead to neurotoxicity and other serious adverse reactions. Therefore, reserve azole antifungals, including Noxafil, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options.
7.11 Calcium Channel Blockers Metabolized by CYP3A4
Noxafil may increase the plasma concentrations of calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, diltiazem, nifedipine, nicardipine, felodipine). Frequent monitoring for adverse reactions and toxicity related to calcium channel blockers is recommended during coadministration. Dose reduction of calcium channel blockers may be needed.
7.12 Digoxin
Increased plasma concentrations of digoxin have been reported in patients receiving digoxin and Noxafil. Therefore, monitoring of digoxin plasma concentrations is recommended during coadministration.
7.14 Glipizide
Although no dosage adjustment of glipizide is required, it is recommended to monitor glucose concentrations when Noxafil and glipizide are concomitantly used.
7.15 Alcohol
Posaconazole was found to release faster from Noxafil PowderMix for delayed-release oral suspension in the presence of alcohol in vitro, which may interfere with its delayed release characteristics. Administration of Noxafil PowderMix for delayed-release oral suspension with alcohol is not recommended [see Clinical Pharmacology (12.3)].
7.16 Venetoclax
Concomitant use of venetoclax (a CYP3A4 substrate) with posaconazole increases venetoclax Cmax and AUC0-INF, which may increase venetoclax toxicities [see Contraindications (4.6), Warnings and Precautions (5.10)]. Refer to the venetoclax prescribing information for more information on the dosing instructions and the extent of increase in venetoclax exposure.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of Noxafil injection, Noxafil delayed-release tablets, Noxafil PowderMix for delayed-release oral suspension, and Noxafil oral suspension for the prophylaxis of invasive Aspergillus and Candida infections have been established in pediatric patients aged 2 and older who are at high risk of developing these infections due to being severely immunocompromised, such as HSCT recipients with GVHD or those with hematologic malignancies with prolonged neutropenia from chemotherapy.
The safety and effectiveness of Noxafil injection and Noxafil delayed-release tablets for the treatment of invasive aspergillosis have been established in pediatric patients aged 13 years and older.
The safety and effectiveness of Noxafil oral suspension have been established for the treatment of oropharyngeal candidiasis (OPC), including OPC refractory (rOPC) to itraconazole and/or fluconazole in pediatric patients aged 13 years and older.
Use of Noxafil in these age groups is supported by evidence from adequate and well-controlled studies of Noxafil in adult and pediatric patients and additional pharmacokinetic and safety data in pediatric patients 2 years of age and older [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14)].
The safety and effectiveness of Noxafil have not been established in pediatric patients younger than 2 years of age.
Noxafil PowderMix for delayed-release oral suspension is not recommended for use in patients who weigh greater than 40 kg because the recommended dosage cannot be achieved with this formulation.
Noxafil PowderMix for delayed-release oral suspension is contraindicated in patients with HFI. Because a diagnosis of HFI may not yet be established in pediatric patients, obtain a careful history of HFI symptoms with sorbitol/fructose/sucrose exposure prior to administration of Noxafil PowderMix for delayed-release oral suspension [see Warnings and Precautions (5.8)].
8.5 Geriatric Use
No overall differences in the safety of Noxafil injection, Noxafil delayed-release tablets, and Noxafil oral suspension were observed between geriatric patients and younger adult patients in the clinical trials; therefore, no dosage adjustment is recommended for any formulation of Noxafil in geriatric patients. No clinically meaningful differences in the pharmacokinetics of Noxafil were observed in geriatric patients compared to younger adult patients during clinical trials [see Clinical Pharmacology (12.3)].
Of the 279 patients treated with Noxafil injection in the Noxafil Injection Study, 52 (19%) were greater than 65 years of age. Of the 230 patients treated with Noxafil delayed-release tablets, 38 (17%) were greater than 65 years of age. Of the 605 patients randomized to Noxafil oral suspension in Noxafil Oral Suspension Study 1 and Study 2, 63 (10%) were ≥65 years of age. In addition, 48 patients treated with greater than or equal to 800-mg/day Noxafil oral suspension in another indication were ≥65 years of age. Of the 288 patients randomized to Noxafil injection/Noxafil delayed-release tablets in the Aspergillosis Treatment Study, 85 (29%) were ≥65 years of age.
No overall differences in the pharmacokinetics and safety were observed between elderly and young subjects during clinical trials, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Following single-dose administration of 400 mg of the Noxafil oral suspension, there was no significant effect of mild (eGFR: 50-80 mL/min/1.73 m2, n=6) or moderate (eGFR: 20-49 mL/min/1.73 m2, n=6) renal impairment on posaconazole pharmacokinetics; therefore, no dose adjustment is required in patients with mild to moderate renal impairment. In subjects with severe renal impairment (eGFR: <20 mL/min/1.73 m2), the mean plasma exposure (AUC) was similar to that in patients with normal renal function (eGFR: >80 mL/min/1.73 m2); however, the range of the AUC estimates was highly variable (CV=96%) in these subjects with severe renal impairment as compared to that in the other renal impairment groups (CV<40%). Due to the variability in exposure, patients with severe renal impairment should be monitored closely for breakthrough fungal infections [see Dosage and Administration (2)]. Similar recommendations apply to Noxafil delayed-release tablets; however, a specific study has not been conducted with the Noxafil delayed-release tablets.
Noxafil injection should be avoided in patients with moderate or severe renal impairment (eGFR <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of Noxafil injection. In patients with moderate or severe renal impairment (eGFR <50 mL/min), receiving the Noxafil injection, accumulation of the intravenous vehicle, SBECD, is expected to occur. Serum creatinine levels should be closely monitored in these patients, and, if increases occur, consideration should be given to changing to oral Noxafil therapy [see Dosage and Administration (2.9) and Warnings and Precautions (5.5)].
8.7 Hepatic Impairment
After a single oral dose of Noxafil oral suspension 400 mg, the mean AUC was 43%, 27%, and 21% higher in subjects with mild (Child-Pugh Class A, N=6), moderate (Child-Pugh Class B, N=6), or severe (Child-Pugh Class C, N=6) hepatic impairment, respectively, compared to subjects with normal hepatic function (N=18). Compared to subjects with normal hepatic function, the mean Cmax was 1% higher, 40% higher, and 34% lower in subjects with mild, moderate, or severe hepatic impairment, respectively. The mean apparent oral clearance (CL/F) was reduced by 18%, 36%, and 28% in subjects with mild, moderate, or severe hepatic impairment, respectively, compared to subjects with normal hepatic function. The elimination half-life (t½) was 27 hours, 39 hours, 27 hours, and 43 hours in subjects with normal hepatic function and mild, moderate, or severe hepatic impairment, respectively.
It is recommended that no dose adjustment of Noxafil oral suspension, Noxafil delayed-release tablets, Noxafil PowderMix for delayed-release oral suspension, and Noxafil injection is needed in patients with mild to severe hepatic impairment (Child-Pugh Class A, B, or C) [see Dosage and Administration (2) and Warnings and Precautions (5.4)]. However, a specific study has not been conducted with Noxafil delayed-release tablets, Noxafil PowderMix for delayed-release oral suspension, and Noxafil injection.
8.8 Gender
The pharmacokinetics of posaconazole are comparable in males and females. No adjustment in the dosage of Noxafil is necessary based on gender.
8.9 Race
The pharmacokinetic profile of posaconazole is not significantly affected by race. No adjustment in the dosage of Noxafil is necessary based on race.
8.10 Weight
Pharmacokinetic modeling suggests that patients weighing greater than 120 kg may have lower posaconazole plasma drug exposure. It is, therefore, suggested to closely monitor for breakthrough fungal infections particularly when using Noxafil oral suspension [see Clinical Pharmacology (12.3)].
10. Overdosage
There is no experience with overdosage of Noxafil injection and Noxafil delayed-release tablets.
During the clinical trials, some patients received Noxafil oral suspension up to 1600 mg/day with no adverse reactions noted that were different from the lower doses. In addition, accidental overdose was noted in one patient who took 1200 mg twice daily Noxafil oral suspension for 3 days. No related adverse reactions were noted by the investigator.
Posaconazole is not removed by hemodialysis.
11. Noxafil Description
Noxafil (posaconazole) is an azole antifungal agent. Noxafil is available as an injection solution to be diluted before intravenous administration, delayed-release tablet, oral suspension, and for delayed-release oral suspension intended for oral administration. Noxafil PowderMix for delayed-release oral suspension must be reconstituted before oral administration.
Posaconazole is designated chemically as 4-[4-[4-[4-[[ (3R,5R)-5- (2,4-difluorophenyl)tetrahydro-5- (1H-1,2,4-triazol-1-ylmethyl)-3-furanyl]methoxy]phenyl]-1-piperazinyl]phenyl]-2-[(1S,2S)-1-ethyl-2-hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one with an empirical formula of C37H42F2N8O4 and a molecular weight of 700.8. The chemical structure is:
 |
Posaconazole is a white powder with a low aqueous solubility.
Noxafil Injection
Noxafil injection is available as a clear colorless to yellow, sterile liquid essentially free of foreign matter. Each vial contains 300 mg of posaconazole and the following inactive ingredients: 6.68 g Betadex Sulfobutyl Ether Sodium (SBECD), 0.0033 g edetate disodium, hydrochloric acid and sodium hydroxide to adjust the pH to 2.6, and water for injection.
Noxafil Delayed-Release Tablets
Noxafil delayed-release tablet is a yellow, coated, oblong tablet containing 100 mg of posaconazole. Each delayed-release tablet contains the inactive ingredients: croscarmellose sodium, hydroxypropylcellulose, hypromellose acetate succinate, iron oxide yellow, Macrogol/PEG 3350, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol partially hydrolyzed, silicon dioxide, talc, and titanium dioxide.
Noxafil Oral Suspension
Noxafil oral suspension is a white, cherry-flavored immediate-release suspension containing 40 mg of posaconazole per mL and the following inactive ingredients: artificial cherry flavor, citric acid monohydrate, glycerin, liquid glucose, polysorbate 80, purified water, simethicone, sodium benzoate, sodium citrate dihydrate, titanium dioxide, and xanthan gum.
Noxafil PowderMix for Delayed-Release Oral Suspension
Noxafil PowderMix for delayed-release oral suspension is supplied as a component of a kit. Each kit contains Noxafil as an off-white to yellowish powder for delayed-release oral suspension, a bottle of mixing liquid, two 3 mL (green) notched tip syringes, two 10 mL (blue) notched tip syringes, two mixing cups, and one bottle adapter for the mixing liquid bottle. Noxafil PowderMix for delayed-release oral suspension contains 300 mg of posaconazole and the following inactive ingredient: hypromellose acetate succinate. The mixing liquid contains: anhydrous citric acid, antifoam Af emulsion, berry citrus sweet flavor, carboxymethylcellulose sodium, carrageenan calcium sulfate trisodium phosphate, glycerin, methylparaben, microcrystalline cellulose, potassium sorbate, propylparaben, purified water, sodium citrate, sodium phosphate monobasic monohydrate, sodium saccharin, sorbitol solution, and xanthan gum. Once reconstituted, the Noxafil PowderMix for delayed-release oral suspension will be cloudy and free of clumps.
12. Noxafil - Clinical Pharmacology
12.1 Mechanism of Action
Posaconazole is an azole antifungal agent [see Clinical Pharmacology (12.4)].
12.2 Pharmacodynamics
Exposure Response Relationship Prophylaxis: In clinical studies of neutropenic patients who were receiving cytotoxic chemotherapy for acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) or hematopoietic stem cell transplant (HSCT) recipients with Graft versus Host Disease (GVHD), a wide range of plasma exposures to posaconazole was noted following administration of Noxafil oral suspension. A pharmacokinetic-pharmacodynamic analysis of patient data revealed an apparent association between average posaconazole concentrations (Cavg) and prophylactic efficacy (Table 17). A lower Cavg may be associated with an increased risk of treatment failure, defined as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or occurrence of breakthrough invasive fungal infections.
| Prophylaxis in AML/MDS* | Prophylaxis in GVHD† | |||
|---|---|---|---|---|
| Cavg Range (ng/mL) | Treatment Failure‡ (%) | Cavg Range (ng/mL) | Treatment Failure‡ (%) | |
| Cavg = the average posaconazole concentration when measured at steady state | ||||
|
||||
| Quartile 1 | 90-322 | 54.7 | 22-557 | 44.4 |
| Quartile 2 | 322-490 | 37.0 | 557-915 | 20.6 |
| Quartile 3 | 490-734 | 46.8 | 915-1563 | 17.5 |
| Quartile 4 | 734-2200 | 27.8 | 1563-3650 | 17.5 |
Exposure Response Relationship Treatment of Invasive Aspergillosis:
Across a range of posaconazole plasma minimum concentrations (Cmin, range: 244 to 5663 ng/mL) following administration of Noxafil injection and Noxafil delayed-release tablets in patients treated for invasive aspergillosis in Aspergillosis Treatment Study, there was no association between posaconazole Cmin and treatment efficacy [see Clinical Pharmacology (12.3) and Clinical Studies (14.1)]. Similarly, across a range of population pharmacokinetic model-predicted steady-state plasma average concentrations (Cavg, range: 589 to 6315 ng/mL), there was no association between posaconazole Cavg and treatment efficacy.
12.3 Pharmacokinetics
General Pharmacokinetic Characteristics
Noxafil Injection
Noxafil injection exhibits dose proportional pharmacokinetics after single doses between 200 and 300 mg in healthy volunteers and patients. The mean pharmacokinetic parameters after single doses with Noxafil injection in healthy volunteers and patients are shown in Table 18.
| Dose (mg) | n | AUC0-∞
(ng∙hr/mL) | AUC0-12
(ng∙hr/mL) | Cmax
(ng/mL) | t1/2
(hr) | CL (L/hr) |
|
|---|---|---|---|---|---|---|---|
| AUC0-∞ = Area under the plasma concentration-time curve from time zero to infinity; AUC0-12 = Area under the plasma concentration-time curve from time zero to 12 hr after the first dose on Day 1; Cmax = maximum observed concentration; t½ = terminal phase half-life; CL = total body clearance; N/D = Not Determined | |||||||
| Healthy Volunteers | 200 | 9 | 35400 (50) | 8840 (20) | 2250 (29) | 23.6 (23) | 6.5 (32) |
| 300 | 9 | 46400 (26) | 13000 (13) | 2840 (30) | 24.6 (20) | 6.9 (27) | |
| Patients | 200 | 30 | N/D | 5570 (32) | 954 (44) | N/D | N/D |
| 300 | 22 | N/D | 8240 (26) | 1590 (62) | N/D | N/D | |
Table 19 displays the pharmacokinetic parameters of posaconazole in patients following administration of Noxafil injection 300 mg taken once a day for 10 or 14 days following twice daily dosing on Day 1.
| Day | N | Cmax
(ng/mL) | Tmax†
(hr) | AUC0-24
(ng*hr/mL) | Cav (ng/mL) | Cmin
(ng/mL) |
|---|---|---|---|---|---|---|
| AUC0-24 = area under the concentration-time curve over the dosing interval (i.e. 24 hours); Cav= time-averaged concentrations (i.e., AUC0-24h/24hr); | ||||||
| Cmin = POS trough level immediately before a subject received the dose of POS on the day specified in the protocol; Cmax = observed maximum plasma concentration; CV = coefficient of variation, expressed as a percent (%); Day = study day on treatment; Tmax = time of observed maximum plasma concentration. | ||||||
|
||||||
| 10/14 | 49 | 3280 (74) | 1.5 (0.98-4.0) | 36100 (35) | 1500 (35) | 1090 (44) |
Noxafil Delayed-Release Tablets
Noxafil delayed-release tablets exhibit dose proportional pharmacokinetics after single and multiple dosing up to 300 mg. The mean pharmacokinetic parameters of posaconazole at steady state following administration of Noxafil delayed-release tablets 300 mg twice daily on Day 1, then 300 mg once daily thereafter in healthy volunteers and in neutropenic patients who are receiving cytotoxic chemotherapy for AML or MDS or HSCT recipients with GVHD are shown in Table 20.
| N | AUC0-24 hr
(ng∙hr/mL) | Cav†
(ng/mL) | Cmax
(ng/mL) | Cmin
(ng/mL) | Tmax‡
(hr) | t1/2
(hr) | CL/F (L/hr) |
|
|---|---|---|---|---|---|---|---|---|
| CV = coefficient of variation expressed as a percentage (%CV); AUC0-T = Area under the plasma concentration-time curve from time zero to 24 hr; Cmax = maximum observed concentration; Cmin = minimum observed plasma concentration; Tmax = time of maximum observed concentration; t½ = terminal phase half-life; CL/F = Apparent total body clearance | ||||||||
|
||||||||
| Healthy Volunteers | 12 | 51618 (25) | 2151 (25) | 2764 (21) | 1785 (29) | 4 (3-6) | 31 (40) | 7.5 (26) |
| Patients | 50 | 37900 (42) | 1580 (42) | 2090 (38) | 1310 (50) | 4 (1.3-8.3) | - | 9.39 (45) |
Noxafil Oral Suspension
Dose-proportional increases in plasma exposure (AUC) to Noxafil oral suspension were observed following single oral doses from 50 mg to 800 mg and following multiple-dose administration from 50 mg twice daily to 400 mg twice daily in healthy volunteers. No further increases in exposure were observed when the dose of the oral suspension increased from 400 mg twice daily to 600 mg twice daily in febrile neutropenic patients or those with refractory invasive fungal infections.
The mean (%CV) [min-max] Noxafil oral suspension average steady-state plasma concentrations (Cavg) and steady-state pharmacokinetic parameters in patients following administration of 200 mg three times a day and 400 mg twice daily of the oral suspension are provided in Table 21.
| Dose* | Cavg (ng/mL) | AUC† (ng∙hr/mL) | CL/F (L/hr) | V/F (L) | t½ (hr) |
|---|---|---|---|---|---|
| Cavg = the average posaconazole concentration when measured at steady state | |||||
| The variability in average plasma posaconazole concentrations in patients was relatively higher than that in healthy subjects. | |||||
|
|||||
| 200 mg three times a day‡ (n=252) | 1103 (67) [21.5-3650] | ND§ | ND§ | ND§ | ND§ |
| 200 mg three times a day¶ (n=215) | 583 (65) [89.7-2200] | 15,900 (62) [4100-56,100] | 51.2 (54) [10.7-146] | 2425 (39) [828-5702] | 37.2 (39) [19.1-148] |
| 400 mg twice daily# (n=23) | 723 (86) [6.70-2256] | 9093 (80) [1564-26,794] | 76.1 (78) [14.9-256] | 3088 (84) [407-13,140] | 31.7 (42) [12.4-67.3] |
Absorption:
Noxafil Delayed-Release Tablets
When given orally in healthy volunteers, Noxafil delayed-release tablets are absorbed with a median Tmax of 4 to 5 hours. Steady-state plasma concentrations are attained by Day 6 at the 300 mg dose (once daily after twice daily loading dose at Day 1). The absolute bioavailability of the oral delayed-release tablet is approximately 54% under fasted conditions. The Cmax and AUC of posaconazole following administration of Noxafil delayed-release tablets is increased 16% and 51%, respectively, when given with a high fat meal compared to a fasted state (see Table 22).
| Fasting Conditions | Fed Conditions (High Fat Meal)* | Fed/Fasting | |||
|---|---|---|---|---|---|
| Pharmacokinetic Parameter | N | Mean (%CV) | N | Mean (%CV) | GMR (90% CI) |
| GMR=Geometric least-squares mean ratio; CI=Confidence interval | |||||
|
|||||
| Cmax (ng/mL) | 14 | 935 (34) | 16 | 1060 (25) | 1.16 (0.96, 1.41) |
| AUC0-72hr (hr∙ng/mL) | 14 | 26200 (28) | 16 | 38400 (18) | 1.51 (1.33, 1.72) |
| Tmax† (hr) | 14 | 5.00 (3.00, 8.00) | 16 | 6.00 (5.00, 24.00) | N/A |
Concomitant administration of Noxafil delayed-release tablets with drugs affecting gastric pH or gastric motility did not demonstrate any significant effects on posaconazole pharmacokinetic exposure (see Table 23).
| Coadministered Drug | Administration Arms | Change in Cmax
(ratio estimate*; 90% CI of the ratio estimate) | Change in AUC0-last
(ratio estimate*; 90% CI of the ratio estimate) |
|---|---|---|---|
|
|||
| Mylanta® Ultimate strength liquid (Increase in gastric pH) | 25.4 meq/5 mL, 20 mL | ↑6% (1.06; 0.90 -1.26)↑ | ↑4% (1.04; 0.90 -1.20) |
| Ranitidine (Zantac®) (Alteration in gastric pH) | 150 mg (morning dose of 150 mg Ranitidine twice daily) | ↑4% (1.04; 0.88 -1.23)↑ | ↓3% (0.97; 0.84 -1.12) |
| Esomeprazole (Nexium®) (Increase in gastric pH) | 40 mg (every morning for 5 days, Day -4 to 1) | ↑2% (1.02; 0.88-1.17)↑ | ↑5% (1.05; 0.89 -1.24) |
| Metoclopramide (Reglan®) (Increase in gastric motility) | 15 mg four times daily for 2 days (Day -1 and 1) | ↓14% (0.86, 0.73,1.02) | ↓7% (0.93, 0.803,1.07) |
Noxafil PowderMix for Delayed-Release Oral Suspension
The absolute bioavailability of the Noxafil PowderMix for delayed-release oral suspension is approximately 70-80%. The effect of food on the pharmacokinetics of the Noxafil PowderMix for delayed-release oral suspension has not been determined.
Concomitant administration of Noxafil PowderMix for delayed-release oral suspension with drugs affecting gastric pH or gastric motility would not be expected to demonstrate any significant effects on posaconazole pharmacokinetic exposure based on similarity to the delayed-release tablets.
An in vitro dissolution study was conducted to evaluate the impact of alcohol (5, 10, 20, and 40%) on the dissolution of Noxafil PowderMix delayed-release oral suspension. The study showed alcohol-induced dose-dumping potential with the Noxafil PowderMix delayed-release oral suspension [see Dosage and Administration (2.1) and Drug Interactions (7.15)].
Noxafil Oral Suspension
Noxafil oral suspension is absorbed with a median Tmax of ~3 to 5 hours. Steady-state plasma concentrations are attained at 7 to 10 days following multiple-dose administration.
Following single-dose administration of 200 mg, the mean AUC and Cmax of posaconazole are approximately 3-times higher when the oral suspension is administered with a nonfat meal and approximately 4-times higher when administered with a high-fat meal (~50 gm fat) relative to the fasted state. Following single-dose administration of Noxafil oral suspension 400 mg, the mean AUC and Cmax of posaconazole are approximately 3-times higher when administered with a liquid nutritional supplement (14 gm fat) relative to the fasted state (see Table 24). In addition, the effects of varying gastric administration conditions on the Cmax and AUC of Noxafil oral suspension in healthy volunteers have been investigated and are shown in Table 25.
In order to assure attainment of adequate plasma concentrations, it is recommended to administer Noxafil oral suspension during or immediately following a full meal. In patients who cannot eat a full meal, Noxafil oral suspension should be taken with a liquid nutritional supplement or an acidic carbonated beverage (e.g., ginger ale).
| Dose (mg) | Cmax
(ng/mL) | Tmax*
(hr) | AUC (I) (ng∙hr/mL) | CL/F (L/hr) | t½
(hr) |
|---|---|---|---|---|---|
|
|||||
| 200 mg fasted (n=20)† | 132 (50) [45-267] | 3.50 [1.5-36‡] | 4179 (31) [2705-7269] | 51 (25) [28-74] | 23.5 (25) [15.3-33.7] |
| 200 mg nonfat (n=20)† | 378 (43) [131-834] | 4 [3-5] | 10,753 (35) [4579-17,092] | 21 (39) [12-44] | 22.2 (18) [17.4-28.7] |
| 200 mg high fat (54 gm fat) (n=20)† | 512 (34) [241-1016] | 5 [4-5] | 15,059 (26) [10,341-24,476] | 14 (24) [8.2-19] | 23.0 (19) [17.2-33.4] |
| 400 mg fasted (n=23)§ | 121 (75) [27-366] | 4 [2-12] | 5258 (48) [2834-9567] | 91 (40) [42-141] | 27.3 (26) [16.8-38.9] |
| 400 mg with liquid nutritional supplement (14 gm fat) (n=23)§ | 355 (43) [145-720] | 5 [4-8] | 11,295 (40) [3865-20,592] | 43 (56) [19-103] | 26.0 (19) [18.2-35.0] |
| Study Description | Administration Arms | Change in Cmax
(ratio estimate†; 90% CI of the ratio estimate) | Change in AUC (ratio estimate†; 90% CI of the ratio estimate) |
|---|---|---|---|
|
|||
| 400-mg single dose with a high-fat meal relative to fasted state (n=12) | 5 minutes before high-fat meal | ↑96% (1.96; 1.48-2.59) | ↑111% (2.11; 1.60-2.78) |
| During high-fat meal | ↑339% (4.39; 3.32-5.80) | ↑382% (4.82; 3.66-6.35) |
|
| 20 minutes after high-fat meal | ↑333% (4.33; 3.28-5.73) | ↑387% (4.87; 3.70-6.42) |
|
| 400 mg twice daily and 200 mg four times daily for 7 days in fasted state and with liquid nutritional supplement (BOOST®) (n=12) | 400 mg twice daily with BOOST | ↑65% (1.65; 1.29-2.11) | ↑66% (1.66; 1.30-2.13) |
| 200 mg four times daily with BOOST | No Effect | No Effect | |
| Divided daily dose from 400 mg twice daily to 200 mg four times daily for 7 days regardless of fasted conditions or with BOOST (n=12) | Fasted state | ↑136% (2.36; 1.84-3.02) | ↑161% (2.61; 2.04-3.35) |
| With BOOST | ↑137% (2.37; 1.86-3.04) | ↑157% (2.57; 2.00-3.30) |
|
| 400-mg single dose with carbonated acidic beverage (ginger ale) and/or proton pump inhibitor (esomeprazole) (n=12) | Ginger ale | ↑92% (1.92; 1.51-2.44) | ↑70% (1.70; 1.43-2.03) |
| Esomeprazole | ↓32% (0.68; 0.53-0.86) | ↓30% (0.70; 0.59-0.83) |
|
| 400-mg single dose with a prokinetic agent (metoclopramide 10 mg three times a day for 2 days) + BOOST or an antikinetic agent (loperamide 4-mg single dose) + BOOST (n=12) | With metoclopramide + BOOST | ↓21% (0.79; 0.72-0.87) | ↓19% (0.81; 0.72-0.91) |
| With loperamide + BOOST | ↓3% (0.97; 0.88-1.07) | ↑11% (1.11; 0.99-1.25) |
|
| 400-mg single dose either orally with BOOST or via an NG tube with BOOST (n=16) | Via NG tube‡ | ↓19% (0.81; 0.71-0.91) | ↓23% (0.77; 0.69-0.86) |
Concomitant administration of Noxafil oral suspension with drugs affecting gastric pH or gastric motility results in lower posaconazole exposure. (See Table 26.)
| Coadministered Drug (Postulated Mechanism of Interaction) | Coadministered Drug Dose/Schedule | Noxafil Dose/Schedule | Effect on Bioavailability of Posaconazole | |
|---|---|---|---|---|
| Change in Mean Cmax (ratio estimate*; 90% CI of the ratio estimate) | Change in Mean AUC (ratio estimate*; 90% CI of the ratio estimate) |
|||
|
||||
| Cimetidine (Alteration of gastric pH) | 400 mg twice daily × 10 days | 200 mg (tablets) once daily × 10 days† | ↓ 39% (0.61; 0.53-0.70) | ↓ 39% (0.61; 0.54-0.69) |
| Esomeprazole (Increase in gastric pH)‡ | 40 mg every morning × 3 days | 400 mg (oral suspension) single dose | ↓ 46% (0.54; 0.43-0.69) | ↓ 32% (0.68; 0.57-0.81) |
| Metoclopramide (Increase in gastric motility)‡ | 10 mg three times a day × 2 days | 400 mg (oral suspension) single dose | ↓ 21% (0.79; 0.72-0.87) | ↓ 19% (0.81; 0.72-0.91) |
Metabolism:
Posaconazole primarily circulates as the parent compound in plasma. Of the circulating metabolites, the majority are glucuronide conjugates formed via UDP glucuronidation (phase 2 enzymes). Posaconazole does not have any major circulating oxidative (CYP450 mediated) metabolites. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose.
Posaconazole is primarily metabolized via UDP glucuronidation (phase 2 enzymes) and is a substrate for p-glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. A summary of drugs studied clinically with the oral suspension or an early tablet formulation, which affect posaconazole concentrations, is provided in Table 27.
| Coadministered Drug (Postulated Mechanism of Interaction) | Coadministered Drug Dose/Schedule | Noxafil Dose/Schedule | Effect on Bioavailability of Posaconazole | |
|---|---|---|---|---|
| Change in Mean Cmax (ratio estimate*; 90% CI of the ratio estimate) | Change in Mean AUC (ratio estimate*; 90% CI of the ratio estimate) |
|||
|
||||
| Efavirenz (UDP-G Induction) | 400 mg once daily × 10 and 20 days | 400 mg (oral suspension) twice daily × 10 and 20 days | ↓45% (0.55; 0.47-0.66) | ↓ 50% (0.50; 0.43-0.60) |
| Fosamprenavir (unknown mechanism) | 700 mg twice daily × 10 days | 200 mg once daily on the 1st day, 200 mg twice daily on the 2nd day, then 400 mg twice daily × 8 Days | ↓21% 0.79 (0.71-0.89) | ↓23% 0.77 (0.68-0.87) |
| Rifabutin (UDP-G Induction) | 300 mg once daily × 17 days | 200 mg (tablets) once daily × 10 days† | ↓ 43% (0.57; 0.43-0.75) | ↓ 49% (0.51; 0.37-0.71) |
| Phenytoin (UDP-G Induction) | 200 mg once daily × 10 days | 200 mg (tablets) once daily × 10 days† | ↓ 41% (0.59; 0.44-0.79) | ↓ 50% (0.50; 0.36-0.71) |
In vitro studies with human hepatic microsomes and clinical studies indicate that posaconazole is an inhibitor primarily of CYP3A4. A clinical study in healthy volunteers also indicates that posaconazole is a strong CYP3A4 inhibitor as evidenced by a >5-fold increase in midazolam AUC. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole. A summary of the drugs studied clinically, for which plasma concentrations were affected by posaconazole, is provided in Table 28 [see Contraindications (4) and Drug Interactions (7.1) including recommendations].
| Coadministered Drug (Postulated Mechanism of Interaction is Inhibition of CYP3A4 by posaconazole) | Coadministered Drug Dose/Schedule | Noxafil Dose/Schedule | Effect on Bioavailability of Coadministered Drugs | |
|---|---|---|---|---|
| Change in Mean Cmax
(ratio estimate*; 90% CI of the ratio estimate) | Change in Mean AUC (ratio estimate*; 90% CI of the ratio estimate) |
|||
|
||||
| Sirolimus | 2-mg single oral dose | 400 mg (oral suspension) twice daily × 16 days | ↑ 572% (6.72; 5.62-8.03) | ↑ 788% (8.88; 7.26-10.9) |
| Cyclosporine | Stable maintenance dose in heart transplant recipients | 200 mg (tablets) once daily × 10 days† | ↑ cyclosporine whole blood trough concentrations Cyclosporine dose reductions of up to 29% were required |
|
| Tacrolimus | 0.05-mg/kg single oral dose | 400 mg (oral suspension) twice daily × 7 days | ↑ 121% (2.21; 2.01-2.42) | ↑ 358% (4.58; 4.03-5.19) |
| Simvastatin | 40-mg single oral dose | 100 mg (oral suspension) once daily × 13 days | Simvastatin ↑ 841% (9.41, 7.13-12.44) Simvastatin Acid ↑ 817% (9.17, 7.36-11.43) | Simvastatin ↑ 931% (10.31, 8.40-12.67) Simvastatin Acid ↑634% (7.34, 5.82-9.25) |
| 200 mg (oral suspension) once daily × 13 days | Simvastatin ↑ 1041% (11.41, 7.99-16.29) Simvastatin Acid ↑851% (9.51, 8.15-11.10) | Simvastatin ↑ 960% (10.60, 8.63-13.02) Simvastatin Acid ↑ 748% (8.48, 7.04-10.23) |
||
| Midazolam | 0.4-mg single intravenous dose‡ | 200 mg (oral suspension) twice daily × 7 days | ↑ 30% (1.3; 1.13-1.48) | ↑ 362% (4.62; 4.02-5.3) |
| 0.4-mg single intravenous dose‡ | 400 mg (oral suspension) twice daily × 7 days | ↑62% (1.62; 1.41-1.86) | ↑524% (6.24; 5.43-7.16) |
|
| 2-mg single oral dose‡ | 200 mg (oral suspension) once daily × 7 days | ↑ 169% (2.69; 2.46-2.93) | ↑ 470% (5.70; 4.82-6.74) |
|
| 2-mg single oral dose‡ | 400 mg (oral suspension) twice daily × 7 days | ↑ 138% (2.38; 2.13-2.66) | ↑ 397% (4.97; 4.46-5.54) |
|
| Rifabutin | 300 mg once daily x 17 days | 200 mg (tablets) once daily × 10 days† | ↑ 31% (1.31; 1.10-1.57) | ↑ 72% (1.72;1.51-1.95) |
| Phenytoin | 200 mg once daily PO x 10 days | 200 mg (tablets) once daily × 10 days† | ↑ 16% (1.16; 0.85-1.57) | ↑ 16% (1.16; 0.84-1.59) |
| Ritonavir | 100 mg once daily x 14 days | 400 mg (oral suspension) twice daily x 7 days | ↑ 49% (1.49; 1.04-2.15) | ↑ 80% (1.8;1.39-2.31) |
| Atazanavir | 300 mg once daily x 14 days | 400 mg (oral suspension) twice daily x 7 days | ↑ 155% (2.55; 1.89-3.45) | ↑ 268% (3.68; 2.89-4.70) |
| Atazanavir/ ritonavir boosted regimen | 300 mg/100 mg once daily x 14 days | 400 mg (oral suspension) twice daily x 7 days | ↑ 53% (1.53; 1.13-2.07) | ↑ 146% (2.46; 1.93-3.13) |
Additional clinical studies demonstrated that no clinically significant effects on zidovudine, lamivudine, indinavir, or caffeine were observed when administered with Noxafil 200 mg once daily; therefore, no dose adjustments are required for these coadministered drugs when coadministered with Noxafil 200 mg once daily.
Excretion:
Following administration of Noxafil oral suspension, posaconazole is predominantly eliminated in the feces (71% of the radiolabeled dose up to 120 hours) with the major component eliminated as parent drug (66% of the radiolabeled dose). Renal clearance is a minor elimination pathway, with 13% of the radiolabeled dose excreted in urine up to 120 hours (<0.2% of the radiolabeled dose is parent drug).
Noxafil injection is eliminated with a mean terminal half-life (t½) of 27 hours and a total body clearance (CL) of 7.3 L/h.
Noxafil delayed-release tablet is eliminated with a mean half-life (t½) ranging between 26 to 31 hours.
Noxafil oral suspension is eliminated with a mean half-life (t½) of 35 hours (range: 20-66 hours).
Specific Populations
No clinically significant differences in the pharmacokinetics of posaconazole were observed based on age, sex, renal impairment, and indication (prophylaxis or treatment).
Race/Ethnicity:
In a population pharmacokinetic analysis of posaconazole, AUC was found to be 25% higher in Chinese patients relative to patients from other races/ethnicities. This higher exposure is not expected to be clinically relevant given the expected variability in posaconazole exposure.
Patients Weighing More Than 120 kg:
Weight has a clinically significant effect on posaconazole clearance. Relative to 70 kg patients, the Cavg is decreased by 25% in patients greater than 120 kg. Patients administered Noxafil weighing more than 120 kg may be at higher risk for lower posaconazole plasma concentrations compared to lower weight patients [see Use in Specific Populations (8.10)].
Pediatric Patients
The mean pharmacokinetic parameters after multiple-dose administration of Noxafil injection and Noxafil PowderMix for delayed-release oral suspension in neutropenic pediatric patients 2 to less than 18 years of age are shown in Table 29. Patients were enrolled into 2 age groups and received Noxafil injection and Noxafil PowderMix for delayed-release oral suspension doses at 6 mg/kg (0.6 to 1 times the recommended dose) with a maximum 300 mg dose once daily (twice daily on Day 1) [see Adverse Reactions (6.1)].
| Age Group | Dose Type | N | AUC0-24 hr (ng·hr/mL) | Cav†
(ng/mL) | Cmax
(ng/mL) | Cmin
(ng/mL) | Tmax‡
(hr) | CL/F§
(L/hr) |
|---|---|---|---|---|---|---|---|---|
| IV= Noxafil injection; PFS= Noxafil PowderMix for delayed-release oral suspension; AUC0-24 = Area under the plasma concentration-time curve from time zero to 24 hr; Cmax = maximum observed concentration; Cmin = minimum observed plasma concentration; Tmax = time of maximum observed concentration; CL /F = apparent total body clearance | ||||||||
|
||||||||
| 2 to <7 years | IV | 17 | 31100 (48.9) | 1300 (48.9) | 3060 (54.1) | 626 (104.8) | 1.75 (1.57-1.83) | 3.27 (49.3) |
| PFS | 7 | 23000 (47.3) | 960 (47.3) | 1510 (43.4) | 542 (68.8) | 4.00 (2.17-7.92) | 4.60 (35.2) |
|
| 7 to 17 years | IV | 24 | 44200 (41.5) | 1840 (41.5) | 3340 (39.4) | 1160 (60.4) | 1.77 (1.33-6.00) | 4.76 (55.7) |
| PFS | 12 | 25000 (184.3) | 1040 (184.3) | 1370 (178.5) | 713 (300.6) | 2.78 (0.00-4.00) | 8.39 (190.3) |
|
Based on a population pharmacokinetic model evaluating posaconazole pharmacokinetics and predicting exposures in pediatric patients, the exposure of steady-state posaconazole average concentration greater than or equal to 700 ng/mL in approximately 90% of patients is attained with the recommended dose of Noxafil injection and Noxafil PowderMix for delayed-release oral suspension.
The population pharmacokinetic analysis of posaconazole in pediatric patients suggests that age, sex, renal impairment and ethnicity have no clinically meaningful effect on the pharmacokinetics of posaconazole.
A total of 12 patients 13 to 17 years of age received 600 mg/day (200 mg three times a day) of Noxafil oral suspension for prophylaxis of invasive fungal infections. Based on pharmacokinetic data in 10 of these pediatric patients, the mean steady-state Cav was similar between these patients and adults (≥18 years of age). In a study of 136 neutropenic pediatric patients 11 months to less than 18 years treated with Noxafil oral suspension, the exposure target of steady-state posaconazole Cavg between 500 ng/mL and less than 2500 ng/mL was attained in approximately 50% of patients instead of the pre-specified 90% of patients.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No drug-related neoplasms were recorded in rats or mice treated with posaconazole for 2 years at doses higher than the clinical dose. In a 2-year carcinogenicity study, rats were given posaconazole orally at doses up to 20 mg/kg (females), or 30 mg/kg (males). These doses are equivalent to 3.9- or 3.5-times the exposure achieved with a 400-mg twice daily oral suspension regimen, respectively, based on steady-state AUC in healthy volunteers administered a high-fat meal (400-mg twice daily oral suspension regimen). In the mouse study, mice were treated at oral doses up to 60 mg/kg/day or 4.8-times the exposure achieved with a 400-mg twice daily oral suspension regimen.
Mutagenesis
Posaconazole was not genotoxic or clastogenic when evaluated in bacterial mutagenicity (Ames), a chromosome aberration study in human peripheral blood lymphocytes, a Chinese hamster ovary cell mutagenicity study, and a mouse bone marrow micronucleus study.
Impairment of Fertility
Posaconazole had no effect on fertility of male rats at a dose up to 180 mg/kg (1.7 x the 400-mg twice daily oral suspension regimen based on steady-state plasma concentrations in healthy volunteers) or female rats at a dose up to 45 mg/kg (2.2 x the 400-mg twice daily oral suspension regimen).
13.2 Animal Toxicology and/or Pharmacology
In a nonclinical study using intravenous administration of posaconazole in very young dogs (dosed from 2 to 8 weeks of age), an increase in the incidence of brain ventricle enlargement was observed in treated animals as compared with concurrent control animals. No difference in the incidence of brain ventricle enlargement between control and treated animals was observed following the subsequent 5-month treatment-free period. There were no neurologic, behavioral or developmental abnormalities in the dogs with this finding, and a similar brain finding was not seen with oral posaconazole administration to juvenile dogs (4 days to 9 months of age). There were no drug-related increases in the incidence of brain ventricle enlargement when treated and control animals were compared in a separate study of 10-week old dogs dosed with intravenous posaconazole for 13 weeks with a 9-week recovery period or a follow-up study of 31-week old dogs dosed for 3 months.
14. Clinical Studies
14.1 Treatment of Invasive Aspergillosis with Noxafil Injection and Noxafil Delayed-Release Tablets
Aspergillosis Treatment Study (NCT01782131) was a randomized, double-blind, controlled trial which evaluated the safety and efficacy of Noxafil injection and Noxafil delayed-release tablets versus voriconazole for primary treatment of invasive fungal disease caused by Aspergillus species. Eligible patients had proven, probable, or possible invasive fungal infections per the European Organization for Research and Treatment of Cancer/Mycoses Study Group, EORTC/MSG criteria. Patients were stratified by risk for mortality or poor outcome where high risk included a history of allogeneic bone marrow transplant, liver transplant, or relapsed leukemia undergoing salvage chemotherapy. The median age of patients was 57 years (range 14-91 years), with 27.8% of patients aged ≥65 years; 5 patients were pediatric patients 14-16 years of age, of whom 3 were treated with Noxafil and 2 with voriconazole. The majority of patients were male (59.8%) and white (67.1%). With regard to risk factors for invasive aspergillosis, approximately two-thirds of the patients in the study had a recent history of neutropenia, while approximately 20% with a history of an allogeneic stem cell transplant. Over 80% of subjects in each treatment group had infection limited to the lower respiratory tract (primarily lung), while approximately 11% to 13% also had infection in another organ. Invasive aspergillosis was proven or probable in 58.1% of patients as classified by independent adjudicators blinded to study treatment assignment. At least one Aspergillus species was identified in 21% of the patients; A. fumigatus and A. flavus were the most common pathogens identified.
Patients randomized to receive Noxafil were given a dose of 300 mg once daily (twice daily on Day 1) IV or tablet. Patients randomized to receive voriconazole were given a dose of 6 mg/kg twice daily Day 1 followed by 4 mg/kg twice daily IV, or oral 300 mg twice daily Day 1 followed by 200 mg twice daily. The recommended initial route of administration was IV; however, patients could begin oral therapy if clinically stable and able to tolerate oral dosing. The transition from IV to oral therapy occurred when the patient was clinically stable. The protocol recommended duration of therapy was 84 days with a maximum allowed duration of 98 days. Median treatment duration was 67 days for Noxafil patients and 64 days for voriconazole patients. Overall, 55% to 60% of patients began treatment with the IV formulation with a median duration of 9 days for the initial IV dosing.
The Intent to Treat (ITT) population included all patients randomized and receiving at least one dose of study treatment. All-cause mortality through Day 42 in the overall population (ITT) was 15.3% for Noxafil patients compared to 20.6% for voriconazole patients for an adjusted treatment difference of -5.3% with a 95% confidence interval of -11.6 to 1.0%. Consistent results were seen in patients with proven or probable invasive aspergillosis per EORTC criteria (see Table 30).
| Noxafil Injection and Delayed-Release Tablets | Voriconazole | ||||
|---|---|---|---|---|---|
| Population | N | n (%) | N | n (%) | Difference* (95% CI) |
|
|||||
| Intent to Treat | 288 | 44 (15.3) | 287 | 59 (20.6) | -5.3 (-11.6, 1.0) |
| Proven/Probable Invasive Aspergillosis | 163 | 31 (19.0) | 171 | 32 (18.7) | 0.3 (-8.2, 8.8) |
Global clinical response at Week 6 was assessed by a blinded, independent adjudication committee based upon prespecified clinical, radiologic, and mycologic criteria. In the subgroup of patients with proven or probable invasive aspergillosis per EORTC criteria, the global clinical response of success (complete or partial response) at Week 6 was seen in 44.8% for Noxafil-treated patients compared to 45.6% for voriconazole-treated patients (see Table 31).
| Posaconazole | Voriconazole | ||||
|---|---|---|---|---|---|
| Population | N | Success | N | Success | Difference† (95% CI) |
|
|||||
| Proven/Probable Invasive Aspergillosis | 163 | 73 (44.8) | 171 | 78 (45.6) | -0.6 (-11.2, 10.1) |
14.2 Prophylaxis of Aspergillus and Candida Infections with Noxafil Oral Suspension
Two randomized, controlled studies were conducted using Noxafil as prophylaxis for the prevention of invasive fungal infections (IFIs) among patients at high risk due to severely compromised immune systems.
The first study (Noxafil Oral Suspension Study 1) was a randomized, double-blind trial that compared Noxafil oral suspension (200 mg three times a day) with fluconazole capsules (400 mg once daily) as prophylaxis against invasive fungal infections in allogeneic hematopoietic stem cell transplant (HSCT) recipients with Graft versus Host Disease (GVHD). Efficacy of prophylaxis was evaluated using a composite endpoint of proven/probable IFIs, death, or treatment with systemic antifungal therapy (patients may have met more than one of these criteria). This assessed all patients while on study therapy plus 7 days and at 16 weeks post-randomization. The mean duration of therapy was comparable between the 2 treatment groups (80 days, Noxafil oral suspension; 77 days, fluconazole). Table 32 contains the results from Noxafil Oral Suspension Study 1.
| Posaconazole n=301 | Fluconazole n=299 |
|
|---|---|---|
|
||
| On therapy plus 7 days | ||
| Clinical Failure* | 50 (17%) | 55 (18%) |
| Failure due to: | ||
| Proven/Probable IFI | 7 (2%) | 22 (7%) |
| (Aspergillus) | 3 (1%) | 17 (6%) |
| (Candida) | 1 (<1%) | 3 (1%) |
| (Other) | 3 (1%) | 2 (1%) |
| All Deaths | 22 (7%) | 24 (8%) |
| Proven/probable fungal infection prior to death | 2 (<1%) | 6 (2%) |
| SAF† | 27 (9%) | 25 (8%) |
| Through 16 weeks | ||
| Clinical Failure*,‡ | 99 (33%) | 110 (37%) |
| Failure due to: | ||
| Proven/Probable IFI | 16 (5%) | 27 (9%) |
| (Aspergillus) | 7 (2%) | 21 (7%) |
| (Candida) | 4 (1%) | 4 (1%) |
| (Other) | 5 (2%) | 2 (1%) |
| All Deaths | 58 (19%) | 59 (20%) |
| Proven/probable fungal infection prior to death | 10 (3%) | 16 (5%) |
| SAF† | 26 (9%) | 30 (10%) |
| Event free lost to follow-up§ | 24 (8%) | 30 (10%) |
The second study (Noxafil Oral Suspension Study 2) was a randomized, open-label study that compared Noxafil oral suspension (200 mg 3 times a day) with fluconazole suspension (400 mg once daily) or itraconazole oral solution (200 mg twice a day) as prophylaxis against IFIs in neutropenic patients who were receiving cytotoxic chemotherapy for AML or MDS. As in Noxafil Oral Suspension Study 1, efficacy of prophylaxis was evaluated using a composite endpoint of proven/probable IFIs, death, or treatment with systemic antifungal therapy (Patients might have met more than one of these criteria). This study assessed patients while on treatment plus 7 days and 100 days postrandomization. The mean duration of therapy was comparable between the 2 treatment groups (29 days, posaconazole; 25 days, fluconazole or itraconazole). Table 33 contains the results from Noxafil Oral Suspension Study 2.
| Posaconazole n=304 | Fluconazole/Itraconazole n=298 |
|
|---|---|---|
|
||
| On therapy plus 7 days | ||
| Clinical Failure*,† | 82 (27%) | 126 (42%) |
| Failure due to: | ||
| Proven/Probable IFI | 7 (2%) | 25 (8%) |
| (Aspergillus) | 2 (1%) | 20 (7%) |
| (Candida) | 3 (1%) | 2 (1%) |
| (Other) | 2 (1%) | 3 (1%) |
| All Deaths | 17 (6%) | 25 (8%) |
| Proven/probable fungal infection prior to death | 1 (<1%) | 2 (1%) |
| SAF‡ | 67 (22%) | 98 (33%) |
| Through 100 days postrandomization | ||
| Clinical Failure† | 158 (52%) | 191 (64%) |
| Failure due to: | ||
| Proven/Probable IFI | 14 (5%) | 33 (11%) |
| (Aspergillus) | 2 (1%) | 26 (9%) |
| (Candida) | 10 (3%) | 4 (1%) |
| (Other) | 2 (1%) | 3 (1%) |
| All Deaths | 44 (14%) | 64 (21%) |
| Proven/probable fungal infection prior to death | 2 (1%) | 16 (5%) |
| SAF‡ | 98 (32%) | 125 (42%) |
| Event free lost to follow-up§ | 34 (11%) | 24 (8%) |
In summary, 2 clinical studies of prophylaxis were conducted with the Noxafil oral suspension. As seen in the accompanying tables (Tables 32 and 33), clinical failure represented a composite endpoint of breakthrough IFI, mortality and use of systemic antifungal therapy. In Noxafil Oral Suspension Study 1 (Table 32), the clinical failure rate of posaconazole (33%) was similar to fluconazole (37%), (95% CI for the difference posaconazole–comparator -11.5% to 3.7%) while in Noxafil Oral Suspension Study 2 (Table 33) clinical failure was lower for patients treated with posaconazole (27%) when compared to patients treated with fluconazole or itraconazole (42%), (95% CI for the difference posaconazole–comparator -22.9% to -7.8%).
All-cause mortality was similar at 16 weeks for both treatment arms in Noxafil Oral Suspension Study 1 [POS 58/301 (19%) vs. FLU 59/299 (20%)]; all-cause mortality was lower at 100 days for Noxafil-treated patients in Noxafil Oral Suspension Study 2 [POS 44/304 (14%) vs. FLU/ITZ 64/298 (21%)]. Both studies demonstrated fewer breakthrough infections caused by Aspergillus species in patients receiving Noxafil prophylaxis when compared to patients receiving fluconazole or itraconazole.
14.3 Treatment of Oropharyngeal Candidiasis with Noxafil Oral Suspension
Noxafil Oral Suspension Study 3 was a randomized, controlled, evaluator-blinded study in HIV-infected patients with oropharyngeal candidiasis. Patients were treated with Noxafil or fluconazole oral suspension (both Noxafil and fluconazole were given as follows: 100 mg twice a day for 1 day followed by 100 mg once a day for 13 days).
Clinical and mycological outcomes were assessed after 14 days of treatment and at 4 weeks after the end of treatment. Patients who received at least 1 dose of study medication and had a positive oral swish culture of Candida species at baseline were included in the analyses (see Table 34). The majority of the subjects had C. albicans as the baseline pathogen.
Clinical success at Day 14 (complete or partial resolution of all ulcers and/or plaques and symptoms) and clinical relapse rates (recurrence of signs or symptoms after initial cure or improvement) 4 weeks after the end of treatment were similar between the treatment arms (see Table 34).
Mycologic eradication rates (absence of colony forming units in quantitative culture at the end of therapy, Day 14), as well as mycologic relapse rates (4 weeks after the end of treatment) were also similar between the treatment arms (see Table 34).
| Noxafil | Fluconazole | |
|---|---|---|
| Clinical Success at End of Therapy (Day 14) | 155/169 (91.7%) | 148/160 (92.5%) |
| Clinical Relapse (4 Weeks after End of Therapy) | 45/155 (29.0%) | 52/148 (35.1%) |
| Mycological Eradication (absence of CFU) at End of Therapy (Day 14) | 88/169 (52.1%) | 80/160 (50.0%) |
| Mycological Relapse (4 Weeks after End of Treatment) | 49/88 (55.6%) | 51/80 (63.7%) |
Mycologic response rates, using a criterion for success as a posttreatment quantitative culture with ≤20 colony forming units (CFU/mL) were also similar between the two groups (Noxafil 68.0%, fluconazole 68.1%). The clinical significance of this finding is unknown.
14.4 Noxafil Oral Suspension Treatment of Oropharyngeal Candidiasis Refractory to Treatment with Fluconazole or Itraconazole
Noxafil Oral Suspension Study 4 was a noncomparative study of Noxafil oral suspension in HIV-infected subjects with OPC that was refractory to treatment with fluconazole or itraconazole. An episode of OPC was considered refractory if there was failure to improve or worsening of OPC after a standard course of therapy with fluconazole greater than or equal to 100 mg/day for at least 10 consecutive days or itraconazole 200 mg/day for at least 10 consecutive days and treatment with either fluconazole or itraconazole had not been discontinued for more than 14 days prior to treatment with Noxafil. Of the 199 subjects enrolled in this study, 89 subjects met these strict criteria for refractory infection.
Forty-five subjects with refractory OPC were treated with Noxafil oral suspension 400 mg twice daily for 3 days, followed by 400 mg once daily for 25 days with an option for further treatment during a 3-month maintenance period. Following a dosing amendment, a further 44 subjects were treated with posaconazole 400 mg twice daily for 28 days. The efficacy of Noxafil was assessed by the clinical success (cure or improvement) rate after 4 weeks of treatment. The clinical success rate was 74.2% (66/89). The clinical success rates for both the original and the amended dosing regimens were similar (73.3% and 75.0%, respectively).
16. How is Noxafil supplied
16.1 How Supplied
Noxafil Injection
Noxafil injection is available as a clear, colorless to yellow sterile liquid in single-dose Type I glass vials closed with bromobutyl rubber stopper and aluminum seal (NDC 0085-4331-01) containing 300 mg of posaconazole in 16.7 mL of solution (18 mg of posaconazole per mL).
Noxafil Delayed-Release Tablets
Noxafil delayed-release tablets are available as yellow, coated, oblong, debossed with "100" on one side containing 100 mg of posaconazole. Bottles with child-resistant closures of 60 delayed-release tablets (NDC 0085-4324-02).
Noxafil Oral Suspension
Noxafil oral suspension is available as a white, cherry-flavored suspension in 4-ounce (123 mL) amber glass bottles with child-resistant closures (NDC 0085-1328-01) containing 105 mL of suspension (40 mg of posaconazole per mL).
Supplied with each oral suspension bottle is a plastic dosing spoon calibrated for measuring 2.5-mL and 5-mL doses.
Noxafil PowderMix for Delayed-Release Oral Suspension
Noxafil PowderMix for delayed-release oral suspension is supplied as:
- Package A: a kit with 8 child-resistant single-use packets of Noxafil PowderMix for delayed-release oral suspension 300 mg, two 3 mL (green) notched tip syringes, two 10 mL (blue) notched tip syringes, two mixing cups, one mixing liquid bottle, and one bottle adapter for the mixing liquid bottle.
- Package B: a box of six 3 mL (green) and six 10 mL (blue) notched tip syringes.
- Packages A and B are supplied separately.
NDC 0085-2224-02 unit of use carton with 8 packets.
NDC 0085-2224-01 individual packet.
16.2 Storage and Handling
Noxafil Injection
Noxafil injection vial should be stored refrigerated at 2 to 8°C (36 to 46°F). Storage conditions for the diluted solution are presented in another section of the prescribing information [see Dosage and Administration (2.4)].
Noxafil Delayed-Release Tablets
Store at 20 to 25°C (68 to 77°F), excursions permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature].
Noxafil Oral Suspension
Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F) [see USP Controlled Room Temperature]. DO NOT FREEZE.
Noxafil PowderMix for Delayed-Release Oral Suspension
Store the entire kit at 20 to 25°C (68 to 77°F), excursions permitted to 15 to 30°C (59 to 86°F) in a clean, dry place. Do not open foil packet containing Noxafil PowderMix for delayed-release oral suspension until ready for use. Storage conditions for the reconstituted solution are presented in another section of the prescribing information [see Dosage and Administration (2.8)].
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Important Administration Instructions
Noxafil Delayed-Release Tablets
Advise patients that Noxafil delayed-release tablets must be swallowed whole and not divided, crushed, or chewed.
Instruct patients that if they miss a dose, they should take it as soon as they remember. If they do not remember until it is within 12 hours of the next dose, they should be instructed to skip the missed dose and go back to the regular schedule. Patients should not double their next dose or take more than the prescribed dose.
Noxafil Oral Suspension
Advise patients to take each dose of Noxafil oral suspension during or immediately (i.e., within 20 minutes) following a full meal. In patients who cannot eat a full meal, each dose of Noxafil oral suspension should be administered with a liquid nutritional supplement or an acidic carbonated beverage (e.g., ginger ale) in order to enhance absorption.
Instruct patients that if they miss a dose, they should take it as soon as they remember. However, if it is almost time for the next dose, they should be instructed to skip the missed dose and go back to the regular schedule. Patients should not double their next dose or take more than the prescribed dose.
Noxafil PowderMix for Delayed-Release Oral Suspension
Instruct parents and/or caregivers that ONLY the provided notched tip syringes can be used to administer Noxafil PowderMix for delayed-release oral suspension to pediatric patients.
Advise patients to take Noxafil PowderMix for delayed-release oral suspension with food.
Drug Interactions
Advise patients to inform their physician immediately if they:
- develop severe diarrhea or vomiting.
- are currently taking drugs that are known to prolong the QTc interval and are metabolized through CYP3A4.
- are currently taking a cyclosporine or tacrolimus, or they notice swelling in an arm or leg or shortness of breath.
- are taking other drugs or before they begin taking other drugs as certain drugs can decrease or increase the plasma concentrations of posaconazole.
Serious and Potentially Serious Adverse Reactions
Advise patients to inform their physician immediately if they:
- notice a change in heart rate or heart rhythm or have a heart condition or circulatory disease. Noxafil can be administered with caution to patients with potentially proarrhythmic conditions.
- are pregnant, plan to become pregnant, or are nursing.
- have liver disease or develop itching, nausea or vomiting, their eyes or skin turn yellow, they feel more tired than usual or feel like they have the flu.
- have ever had an allergic reaction to other antifungal medicines such as ketoconazole, fluconazole, itraconazole, or voriconazole.
Hereditary Fructose Intolerance (HFI)
Inform patients and caregivers that Noxafil PowderMix for delayed-release oral suspension contains sorbitol and can be life-threatening when administered to patients with hereditary fructose intolerance (HFI) [see Warnings and Precautions (5.8)]. Inquire for symptoms of sorbitol/fructose and/or sucrose intolerance before administration.
| This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 09/2022 | |||
| Patient Information Noxafil® (NOX-a-fil) (posaconazole) injection Noxafil® (NOX-a-fil) (posaconazole) delayed-release tablets Noxafil® (NOX-a-fil) (posaconazole) oral suspension Noxafil® (NOX-a-fil) PowderMix (posaconazole) for delayed-release oral suspension |
|||
|
What is Noxafil and Noxafil PowderMix?
Noxafil injection is used for:
Noxafil delayed-release tablets are used for:
Noxafil oral suspension is used for:
Noxafil PowderMix for delayed-release oral suspension is used for:
Noxafil oral suspension is also used to treat a fungal infection called “thrush” caused by Candida in your mouth or throat area. Noxafil oral suspension can be used as the first treatment for thrush, or as another treatment for thrush after itraconazole or fluconazole treatment has not worked. Noxafil oral suspension is for adults and children 13 years of age and older. It is not known if Noxafil or Noxafil PowderMix is safe and effective in children under 2 years of age. |
|||
| Who should not take Noxafil or Noxafil PowderMix?
Do not take Noxafil or Noxafil PowderMix if you:
Do not start taking a new medicine without talking to your healthcare provider or pharmacist. |
|||
| What should I tell my healthcare provider before taking Noxafil or Noxafil PowderMix?
Before you take Noxafil or Noxafil PowderMix, tell your healthcare provider if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Noxafil and Noxafil PowderMix can affect the way other medicines work, and other medicines can affect the way Noxafil and Noxafil PowderMix work, and can cause serious side effects.
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure. |
|||
|
How will I take Noxafil or Noxafil PowderMix?
Follow the instructions from your healthcare provider on how much Noxafil or Noxafil PowderMix you should take and when to take it. |
|||
|
What are the possible side effects of Noxafil or Noxafil PowderMix? Noxafil or Noxafil PowderMix may cause serious side effects, including:
|
|||
The most common side effects of Noxafil in adults include: |
|||
|
|
||
|
The most common side effects of Noxafil injection and Noxafil PowderMix in children include: |
|||
|
|
||
|
If you take Noxafil delayed-release tablets, Noxafil oral suspension, or Noxafil PowderMix for delayed-release oral suspension, tell your healthcare provider right away if you have diarrhea or vomiting. |
|||
|
How should I store Noxafil or Noxafil PowderMix? Noxafil injection
Noxafil delayed-release tablets
Noxafil oral suspension
Noxafil PowderMix for delayed-release oral suspension
Safely throw away medicine that is out of date or no longer needed. Keep Noxafil and Noxafil PowderMix and all medicines out of the reach of children. |
|||
|
General information about the safe and effective use of Noxafil and Noxafil PowderMix. |
|||
|
What are the ingredients in Noxafil and Noxafil PowderMix?
|
|||
|
Manuf. for: Merck Sharp & Dohme LLC |
|||
| Important: Read This Booklet First
Noxafil PowderMix (posaconazole) for delayed-release oral suspension Instructions for Use for caregivers and healthcare providers of toddlers and children |
 |
| Before You Start |
|  |
| Note: Put your child in a safe place. You will need both hands to prepare Noxafil PowderMix. Wash your hands with soap and water before preparing Noxafil PowderMix. |
| Before You Start |
- The amount of Noxafil PowderMix you give depends on your child’s weight. Your healthcare provider will tell you the right dose to give your child. Be sure to keep your healthcare provider’s appointments so you get new dosing information as your child grows.
- This booklet tells you how to:
- Make the Noxafil PowderMix into a liquid form.
- Measure the right dose using the included notched-tip
oral syringe. Only use the notched-tip syringes supplied
with the kit. Do not use other oral syringes. - Give the Noxafil PowderMix to your child.
- Clean up.
| Note before adding Noxafil PowderMix: Make sure you and your child are ready! If you do not use Noxafil PowderMix within 1 hour, you will need to throw it away and start over. |
| Note: If you have any questions, call your healthcare provider. |
|  |
|  |
|  |
|  |
|
|  |
|
|
|  |
 |  |
| 2 blue (10 mL) syringes | 2 green (3 mL) syringes |
| The kit has an extra cup and set of syringes in case one is lost or damaged.
Do not use any damaged cups or syringes. |
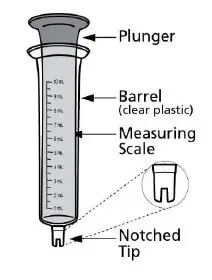 |
|
- Look for the number on the measuring scale that matches
the amount of mixing liquid or Noxafil PowderMix that
you need. - Be sure to follow the directions in this booklet to remove
air bubbles from the syringe. Air bubbles can affect the
amount of medicine that the child gets.
| Step 1. Get the mixing liquid ready. |
| Important: Noxafil PowderMix needs to be prepared using the mixing liquid. Do not mix Noxafil PowderMix with milk, juice, or water. |
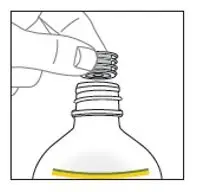 | 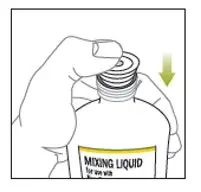 | 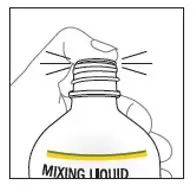 |
When you use the mixing liquid for the first time:
- Open the bottle and remove the safety seal.
- Place the bottle adapter on top of the bottle with
the small hole facing up. - Push the bottle adapter all the way down.
- After it is in place, the bottle adapter stays in the bottle.
- Put the cap back on the bottle.
| Step 2. Gather all your supplies and put on a clean surface. |
| Note: Put your child in a safe place. You will need both hands to prepare Noxafil PowderMix. Wash your hands with soap and water before preparing Noxafil PowderMix. |
 |  |  | 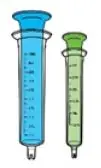 |  |
| 1 mixing cup
(Using the tab on the mixing cup, pull open the lid.) | 1 packet of Noxafil PowderMix powder | Mixing liquid | 2 syringes
(Have 1 of each size ready, but you may only use 1, depending on the dose.) | Scissors
(not included with kit: use sharp household scissors or kitchen scissors) |
 |
The Noxafil PowderMix box has a mixing cup holder inside to help tilt the cup when you are measuring the dose. |
| Step 3. Add Noxafil PowderMix to the mixing cup. |
| Note before adding Noxafil PowderMix:
Make sure you and your child are ready! If you do not use Noxafil PowderMix within 1 hour, you will need to throw it away and start over. |
|  |
| 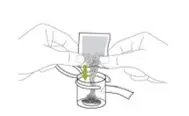 |
| Step 4. Shake the mixing liquid bottle. |
 |
|
| Step 5. Fill the blue syringe with 9 mL of mixing liquid. |
| 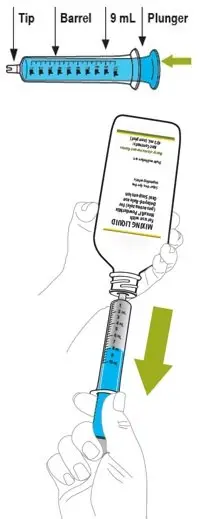 |
| Step 6. Check for air bubbles. |
|
|
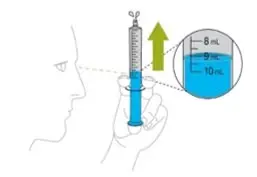 |
|
| Step 7. Add the 9 mL of mixing liquid to the Noxafil PowderMix. |
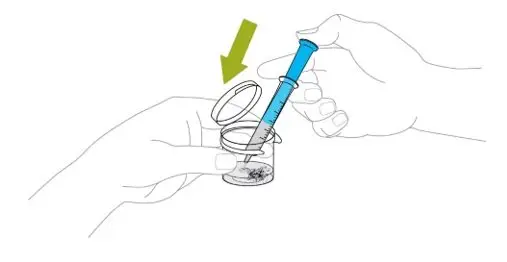 |
|
| Step 8. Mix the Noxafil PowderMix. |
|
|
 |
|
| Step 9. Check your prescription label. |
|
 |
| Note: The dose may change each time you go to the healthcare provider, so make sure you have all the recent information. Be sure to go to all of your child's healthcare provider appointments so your child gets the right dose. |
| Step 10. Choose the syringe you need. |
| Note: Only use the syringes provided in the kit. |
Choose the correct syringe for your child's dose:
| For 1 mL
to 3 mL Green |  | For 3 mL
to 10 mL Blue |  |
- Then find the mL mark on the syringe that matches your
child's dose.
| Step 11. Measure the Noxafil PowderMix. |
|  |
| Important: You will not use all of the Noxafil PowderMix. There will be some left over in the mixing cup. |
| Step 12. Check for air bubbles. |
|
|
 |
|
| Step 13. Give the Noxafil PowderMix to your child. |
|  |
Important:
|
| Step 14. Clean the cup and syringes. |
| Note: Syringes and mixing cups should be reused. Do not throw away syringes and mixing cups provided until all the Noxafil PowderMix packets are used. |
|  |
| Step 15. After all packets of Noxafil PowderMix have been used. |
- After you have used the last Noxafil PowderMix packet
in this box, you will have leftover mixing liquid in the
bottle. Throw away the leftover mixing liquid and all
components of the kit.
How should I store Noxafil PowderMix?
- Store the entire kit at room temperature between 68°F to
77°F (20°C to 25°C) in a clean, dry place. - Keep the mixing liquid at room temperature.
- Do not open the Noxafil PowderMix packet until you are
ready to mix a dose.
Keep NOXAFIL PowderMix and all medicines out of the reach of children.
For more information go to www.Noxafil.com or call
1-800-622-4477.
Distributed by:
Merck Sharp & Dohme LLC
Rahway, NJ 07065, USA
For patent information: www.msd.com/research/patent
Copyright © 2021-2022 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.
ifu-mk5592-2209r002
This Instructions for Use has been approved by the U.S. Food and
Drug Administration.
Revised: September 2022
| NOXAFIL
posaconazole suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| NOXAFIL
posaconazole tablet, coated |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| NOXAFIL
posaconazole solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NOXAFIL
posaconazole powder, for suspension |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |