Drug Detail:Nuzyra (oral/injection) (Omadacycline (oral/injection) [ oh-ma-da-sye-kleen ])
Drug Class: Tetracyclines
Highlights of Prescribing Information
NUZYRA (omadacycline) for injection, for intravenous use
NUZYRA (omadacycline) tablets, for oral use
Initial U.S. Approval: 2018
Recent Major Changes
| Dosage and Administration, Dosage in Adults with Community-Acquired Bacterial Pneumonia (CABP) (2.2) | 5/2021 |
Indications and Usage for Nuzyra
NUZYRA is a tetracycline class antibacterial indicated for the treatment of adult patients with the following infections caused by susceptible microorganisms (1):
- Community-acquired bacterial pneumonia (CABP) (1.1)
- Acute bacterial skin and skin structure infections (ABSSSI) (1.2)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of NUZYRA and other antibacterial drugs, NUZYRA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. (1.3)
Nuzyra Dosage and Administration
- Dosage of NUZYRA in CABP and ABSSSI Adult Patients (2.2, 2.3):
| Infection | Loading Doses | Maintenance Dose |
|---|---|---|
| CABP | NUZYRA Injection:
Day 1: 200 mg by intravenous infusion over 60 minutes OR 100 mg by intravenous infusion over 30 minutes twice (2.2) OR NUZYRA Tablets: Day 1: 300 mg orally twice (2.2) | NUZYRA Injection:
100 mg by intravenous infusion over 30 minutes once daily OR NUZYRA Tablets: 300 mg orally once daily(2.2) |
| ABSSSI | NUZYRA Injection:
Day 1: 200 mg by intravenous infusion over 60 minutes OR 100 mg by intravenous infusion over 30 minutes twice (2.3) OR NUZYRA Tablets: Day 1 and Day 2: 450 mg orally once daily (2.3) | NUZYRA Injection:
100 mg by intravenous infusion over 30 minutes once daily OR NUZYRA Tablets: 300 mg orally once daily (2.3) |
- CABP and ABSSSI: Treatment duration is 7 to 14 days. (2.2, 2.3)
- Fast for at least 4 hours and then take NUZYRA tablets with water. After oral dosing, no food or drink (except water) is to be consumed for 2 hours and no dairy products, antacids, or multivitamins for 4 hours. (2.1)
- See full prescribing information for the preparation of NUZYRA IV and other administration instructions. (2.1, 2.5).
Dosage Forms and Strengths
- For Injection: 100 mg of omadacycline (equivalent to 131 mg omadacycline tosylate) as a lyophilized powder in a single dose vial for reconstitution and further dilution before intravenous infusion (3.1)
- Tablets: 150 mg omadacycline (equivalent to 196 mg omadacycline tosylate) (3.2)
Contraindications
Known hypersensitivity to omadacycline, tetracycline-class antibacterial drugs or any of the excipients in NUZYRA (4)
Warnings and Precautions
- Mortality Imbalance in Patients with CABP: In the CABP trial, mortality rate of 2% was observed in NUZYRA-treated patients compared to 1% in moxifloxacin-treated patients. The cause of the mortality imbalance has not been established. Closely monitor clinical response to therapy in CABP patients, particularly in those at higher risk for mortality. (5.1, 6.1)
- Tooth Discoloration and Enamel Hypoplasia: The use of NUZYRA during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown) and enamel hypoplasia. (5.2, 8.1, 8.4)
- Inhibition of Bone Growth: The use of NUZYRA during the second and third trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth. (5.3, 8.1, 8.4).
- Clostridioides difficile-associated diarrhea: Evaluate if diarrhea occurs. (5.5)
Adverse Reactions/Side Effects
The most common adverse reactions (incidence ≥2%) are nausea, vomiting, infusion site reactions, alanine aminotransferase increased, aspartate aminotransferase increased, gamma-glutamyl transferase increased, hypertension, headache, diarrhea, insomnia, and constipation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Paratek Pharmaceuticals, Inc. at 1-833-727-2835 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage while taking NUZYRA. (7.1)
- Absorption of tetracyclines, including NUZYRA is impaired by antacids containing aluminum, calcium, or magnesium, bismuth subsalicylate and iron containing preparations. (2.1, 7.2)
Use In Specific Populations
Lactation: Breastfeeding is not recommended during treatment with NUZYRA. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2021
Full Prescribing Information
1. Indications and Usage for Nuzyra
1.1 Community-Acquired Bacterial Pneumonia (CABP)
NUZYRA is indicated for the treatment of adult patients with community-acquired bacterial pneumonia (CABP) caused by the following susceptible microorganisms: Streptococcus pneumoniae, Staphylococcus aureus (methicillin-susceptible isolates), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydophila pneumoniae.
1.2 Acute Bacterial Skin and Skin Structure Infections (ABSSSI)
NUZYRA is indicated for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) caused by the following susceptible microorganisms: Staphylococcus aureus (methicillin-susceptible and -resistant isolates), Staphylococcus lugdunensis, Streptococcus pyogenes, Streptococcus anginosus grp. (includes S. anginosus, S. intermedius, and S. constellatus), Enterococcus faecalis, Enterobacter cloacae, and Klebsiella pneumoniae.
1.3 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of NUZYRA and other antibacterial drugs, NUZYRA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2. Nuzyra Dosage and Administration
2.2 Dosage in Adults with Community-Acquired Bacterial Pneumonia (CABP)
For treatment of adults with CABP the recommended dosage regimen (loading and maintenance) of NUZYRA is described in Table 1 below.
| Loading Doses | Maintenance Dose | Treatment Duration |
|---|---|---|
| NUZYRA Injection:
200 mg by intravenous infusion over 60 minutes on day 1. OR 100 mg by intravenous infusion over 30 minutes, twice on day 1. OR | NUZYRA Injection:
100 mg by intravenous infusion over 30 minutes once daily. OR NUZYRA Tablets: 300 mg orally once daily. | 7 to 14 Days |
| NUZYRA Tablets:
300 mg orally twice on day 1. |
2.3 Dosage in Adults with Acute Bacterial Skin Structure and Skin Infections (ABSSSI)
For treatment of adults with ABSSSI, the recommended dosage regimen (loading and maintenance) of NUZYRA is described in Table 2 below.
| Loading Doses | Maintenance Dose | Treatment Duration |
|---|---|---|
| NUZYRA Injection:
200 mg by intravenous infusion over 60 minutes on day 1. OR 100 mg by intravenous infusion over 30 minutes, twice on day 1. OR | NUZYRA Injection:
100 mg by intravenous infusion over 30 minutes once daily. OR NUZYRA Tablets: 300 mg orally once daily. | 7 to 14 Days |
| NUZYRA Tablets:
450 mg orally once a day on day 1 and day 2. |
2.4 Dosage Adjustments in Patients with Renal or Hepatic Impairment
No dosage adjustment is warranted in patients with renal or hepatic impairment [see Clinical Pharmacology (12.3)].
2.5 Preparation and Administration of NUZYRA for Injection Intravenous Solution
Reconstitution and Dilution:
- 1)
- NUZYRA must be reconstituted and then further diluted under aseptic conditions. To prepare the required dose for intravenous infusion, reconstitute and dilute the appropriate number of vials, as determined from Table 3 below.
- 2)
- Reconstitute each 100 mg vial of NUZYRA with 5 mL of Sterile Water, 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP, for Injection.
- 3)
- Gently swirl the contents and let the vial stand until the cake has completely dissolved and any foam disperses. Do not shake the vial.
- 4)
- The reconstituted NUZYRA solution should be yellow to dark orange in color; if not, the solution should be discarded. Visually inspect the reconstituted NUZYRA solution for particulate matter and discoloration prior to further dilution and administration. If necessary, invert the vial to dissolve any remaining powder and swirl gently to prevent foaming.
- 5)
- Immediately (within 1 hour), withdraw 5 mL or 10 ml of the reconstituted solution and further dilute to a 100 mL (nominal volume) of 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP, bag for injection. The concentration of the final diluted infusion solution will either be 1 mg/mL or 2 mg/mL in accordance with Table 3 below. Discard any unused portion of the reconstituted solution.
- 6)
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
| NUZYRA for Injection Dose | Number of Vials to Reconstitute for Further Dilution | Volume of Reconstituted Solution (5 mL/vial) to Withdraw for Further Dilution | Final Infusion Concentration of NUZYRA |
|---|---|---|---|
| 200 mg | 2 Vials | 10 mL | 2 mg/mL |
| 100 mg | 1 Vial | 5 mL | 1 mg/mL |
3. Dosage Forms and Strengths
4. Contraindications
NUZYRA is contraindicated in patients with known hypersensitivity to omadacycline or tetracycline-class antibacterial drugs, or to any of the excipients [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
5. Warnings and Precautions
5.1 Mortality Imbalance in Patients with Community-Acquired Bacterial Pneumonia
Mortality imbalance was observed in the CABP clinical trial with eight deaths (2%) occurring in patients treated with NUZYRA compared to four deaths (1%) in patients treated with moxifloxacin. The cause of the mortality imbalance has not been established.
All deaths, in both treatment arms, occurred in patients > 65 years of age; most patients had multiple comorbidities [see Use in Specific Populations (8.5)]. The causes of death varied and included worsening and/or complications of infection and underlying conditions. Closely monitor clinical response to therapy in CABP patients, particularly in those at higher risk for mortality [see Adverse Reactions (6.1)].
5.2 Tooth Discoloration and Enamel Hypoplasia
The use of NUZYRA during tooth development (last half of pregnancy, infancy, and childhood up to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). This adverse reaction is more common during long-term use of the tetracycline class drugs, but it has been observed following repeated short-term courses. Enamel hypoplasia has also been reported with tetracycline class drugs. Advise the patient of the potential risk to the fetus if NUZYRA is used during the second or third trimester of pregnancy [see Use in Specific Populations (8.1, 8.4)].
5.3 Inhibition of Bone Growth
The use of NUZYRA during the second and third trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth. All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature infants given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued. Advise the patient of the potential risk to the fetus if NUZYRA is used during the second or third trimester of pregnancy [see Use in Specific Populations (8.1, 8.4)].
5.4 Hypersensitivity Reactions
Hypersensitivity reactions have been reported with NUZYRA [see Adverse Reactions (6.1)]. Life-threatening hypersensitivity (anaphylactic) reactions have been reported with other tetracycline-class antibacterial drugs. NUZYRA is structurally similar to other tetracycline-class antibacterial drugs and is contraindicated in patients with known hypersensitivity to tetracycline-class antibacterial drugs [see Contraindications (4)]. Discontinue NUZYRA if an allergic reaction occurs.
5.5 Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.6 Tetracycline Class Effects
NUZYRA is structurally similar to tetracycline-class of antibacterial drugs and may have similar adverse reactions. Adverse reactions including photosensitivity, pseudotumor cerebri, and anti-anabolic action which has led to increased BUN, azotemia, acidosis, hyperphosphatemia, pancreatitis, and abnormal liver function tests, have been reported for other tetracycline-class antibacterial drugs, and may occur with NUZYRA. Discontinue NUZYRA if any of these adverse reactions are suspected.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described in greater detail in the Warnings and Precautions section of labeling:
- Mortality Imbalance in Patients with Community-Acquired Bacterial Pneumonia [see Warnings and Precautions (5.1)]
- Tooth Development and Enamel Hypoplasia [see Warnings and Precautions (5.2)]
- Inhibition of Bone Growth [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Tetracycline Class Effects [see Warnings and Precautions (5.6]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trial Experience in Patients with Community-Acquired Bacterial Pneumonia
Trial 1 was a Phase 3 CABP trial that enrolled 774 adult patients, 386 randomized to NUZYRA (382 received at least one dose of NUZYRA and 4 patients did not receive the study drug) and 388 randomized to moxifloxacin (all 388 received at least one dose of moxifloxacin). The mean age of patients treated with NUZYRA was 61 years (range 19 to 97 years) and 42% were greater than or equal to 65 years of age. Overall, patients treated with NUZYRA were predominantly male (53.7%), white (92.4%), and had a mean body mass index (BMI) of 27.3 kg/m2. Approximately 47% of NUZYRA treated patients had CrCl <90 ml/min. Patients were administered an IV to oral switch dosage regimen of NUZYRA. The total treatment duration was 7 to 14 days. Mean duration of IV treatment was 5.7 days and mean total duration of treatment was 9.6 days in both treatment arms.
Most Common Adverse Reactions
Table 4 lists the most common adverse reactions occurring in ≥2% of patients receiving NUZYRA in Trial 1.
| Adverse Reaction | NUZYRA (N = 382) | Moxifloxacin (N = 388) |
|---|---|---|
| Alanine aminotransferase increased | 3.7 | 4.6 |
| Hypertension | 3.4 | 2.8 |
| Gamma-glutamyl transferase increased | 2.6 | 2.1 |
| Insomnia | 2.6 | 2.1 |
| Vomiting | 2.6 | 1.5 |
| Constipation | 2.4 | 1.5 |
| Nausea | 2.4 | 5.4 |
| Aspartate aminotransferase increased | 2.1 | 3.6 |
| Headache | 2.1 | 1.3 |
Clinical Trials Experience in Patients with Acute Bacterial Skin and Skin Structure Infections
Trial 2 was a Phase 3 ABSSSI trial that enrolled 655 adult patients, 329 randomized to NUZYRA and 326 randomized to linezolid. Trial 3 was a Phase 3 ABSSSI trial that enrolled 735 adult patients, 368 randomized to NUZYRA and 367 randomized to linezolid.
In Trial 2 (IV to oral switch trial), the mean age of patients treated with NUZYRA was 47 years (range 19 to 88). Overall, patients treated with NUZYRA were predominantly male (62.8%), white (91.0%) and had a mean BMI of 28. kg/m2.
In Trial 3 (oral only trial), the mean age of patients was 43 years (range 18 to 86). Patients treated with NUZYRA were predominantly male (65.8%), white (88.9%), and had a mean BMI of 27.9 kg/m2.
In Trials 2 and 3, approximately 12% of NUZYRA treated patients had CrCl <90 ml/min. Overall, the mean and median calculated lesion area was similar across both trials. Trial 2 required at least 3 days of IV treatment followed by switch to oral regimen based on physician's discretion. Mean duration of IV treatment in Trial 2 was 4 days and mean total duration of treatment was 9 days in both treatment arms. In Trial 3, only oral therapy was administered, and mean total duration of treatment was 8 days in both treatment arms. The median days on treatment in the pooled ABSSSI trials was 9 days for both NUZYRA and linezolid.
Most Common Adverse Reactions
Table 5 includes the most common adverse reactions occurring in ≥2% of patients receiving NUZYRA in Trials 2 and 3.
| Adverse Reaction | NUZYRA (N = 691) | Linezolid (N = 689) |
|---|---|---|
|
||
| Nausea* | 21.9 | 8.7 |
| Vomiting | 11.4 | 3.9 |
| Infusion site reactions† | 5.2 | 3.6 |
| Alanine aminotransferase increased | 4.1 | 3.6 |
| Aspartate aminotransferase increased | 3.6 | 3.5 |
| Headache | 3.3 | 3.0 |
| Diarrhea | 3.2 | 2.9 |
7. Drug Interactions
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of NUZYRA in pediatric patients below the age of 18 years have not been established.
Due to the adverse effects of the tetracycline-class of drugs, including NUZYRA on tooth development and bone growth, use of NUZYRA in pediatric patients less than 8 years of age is not recommended [see Warnings and Precautions (5.1, 5.2)]
8.5 Geriatric Use
Of the total number of patients who received NUZYRA in the Phase 3 clinical trials (n=1073), 200 patients were ≥ 65 years of age, including 92 patients who were ≥75 years of age. In Trial 1, numerically lower clinical success rates at early clinical response (ECR) timepoint for NUZYRA-treated and moxifloxacin-treated patients (75.5% and 78.7%, respectively) were observed in CABP patients ≥ 65 years of age as compared to patients <65 years of age (85.2% and 86.3%, respectively). Additionally, all deaths in the CABP trial occurred in patients >65 years of age [see Adverse Reactions (6.1)].
No significant difference in NUZYRA exposure was observed between healthy elderly subjects and younger subjects following a single 100-mg IV dose of NUZYRA [see Clinical Pharmacology (12.3)].
10. Overdosage
No specific information is available on the treatment of overdosage with NUZYRA. Following a 100 mg single dose intravenous administration of omadacycline, 8.9% of dose is recovered in the dialysate.
11. Nuzyra Description
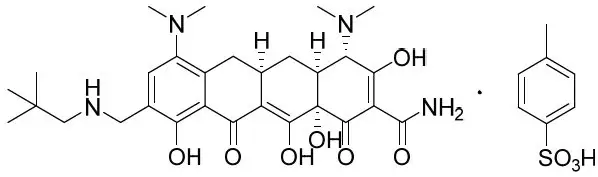
NUZYRA contains omadacycline tosylate, an aminomethylcycline which is a semisynthetic derivative of the tetracycline class of antibacterial drugs, for intravenous or oral administration. The chemical name of omadacycline tosylate is (4S,4aS,5aR,12aS)-4,7-bis(dimethylamino)-9-(2,2-dimethylpropylaminomethyl)-3,10,12,12a-tetrahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide, 4-methylbenzenesulfonate.
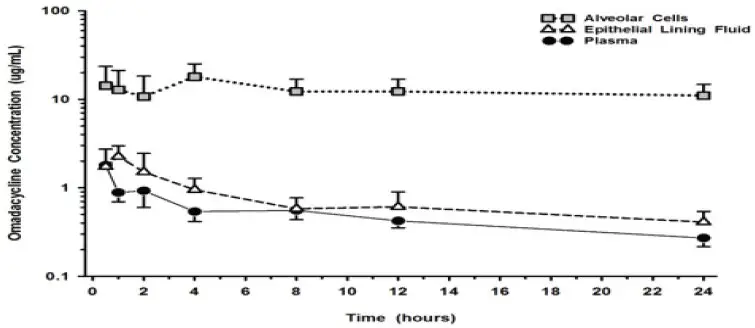
The molecular formula is C36H48N4O10S (monotosylate salt) and the molecular weight is 728.9 (monotosylate salt). The following represents the chemical structure of omadacycline tosylate:
NUZYRA (omadacycline) for injection is a yellow to dark orange sterile lyophilized powder. Each vial of NUZYRA for injection contains 100 mg of omadacycline (equivalent to 131 mg omadacycline tosylate). Inactive ingredients: Sucrose (100 mg); may include hydrochloric acid and/or sodium hydroxide for pH adjustment.
NUZYRA (omadacycline) tablets for oral administration are yellow film coated tablets containing 150 mg of omadacycline (equivalent to 196 mg omadacycline tosylate), and the following inactive ingredients: Colloidal silicon dioxide, crospovidone, glycerol monocaprylocaprate, iron oxide yellow, lactose monohydrate, microcrystalline cellulose, polyvinyl alcohol, sodium bisulfite, sodium lauryl sulfate, sodium stearyl fumarate, talc, and titanium dioxide.
12. Nuzyra - Clinical Pharmacology
12.3 Pharmacokinetics
The pharmacokinetic parameters of NUZYRA after single and multiple oral and intravenous doses are summarized in Table 6.
| Dose and Route of Administration | 100 mg IV | 300 mg Oral | 450 mg Oral | |
|---|---|---|---|---|
| Cmax = maximum plasma concentration, AUC = area under concentration-time curve, IV = intravenous, ND = not determined, Tmax = time to Cmax | ||||
|
||||
| PK Parameters* | ||||
| Cmax ng/mL | Single dose | 1507 (582) (n=63) | 548 (146) (n=103) | 874 (232) (n=24) |
| Steady state | 2116 (680) (n=41) | 952 (420) (n=43) | 1077 (269) (n=24) |
|
| AUC h*ng/mL | Single dose† | 9358 (2072) (n=62) | 9399 (2559) (n=102) | 13504 (3634) (n=24) |
| Steady state‡ | 12140 (3223) (n=41) | 11156 (5010) (n=43) | 13367 (3469) (n=24) |
|
| Accumulation | Accumulation ratio 1.5 | |||
| Absorption | ||||
| Bioavailability | 34.5% following single 300 mg dose of NUZYRA | |||
| Tmax Median (min, max) | Single dose | 0.6 (0.3, 0.7) (n=63) | 2.5 (1, 4.1) (n=103) | 2.5 (1.5, 3) (n=24) |
| Steady state | 0.5 (0, 1) (n=41) | 2.5 (0, 8) (n=43) | 2.5 (1.5, 4) (n=24) |
|
| Distribution | ||||
| Plasma Protein Binding | 20%; not concentration dependent | |||
| Volume of Distribution L | Single dose | 256 (66) (n=62) | 794§ (188) (n=27) | 914§ (821.9) (n=23) |
| Steady state | 190 (53) (n=41) | 440§ (262) (n=34) | 607§ (197.4) (n=24) |
|
| Elimination | ||||
| Elimination Half-Life h | Single dose | 16.4 (2.1) (n=62) | 15.0 (2.5) (n=81) | 13.45 (1.7) (n=23) |
| Steady state | 16.0 (3.5) (n=41) | 15.5 (1.7) (n=21) | 16.83 (1.4) (n=23) |
|
| Systemic Clearance L/h | Single dose | 11.24 (2.7) (n=62) | 34.6§ (10.7) (n=27) | 43.4§ (49.8) (n=23) |
| Steady state | 8.8 (2.2) (n=41) | 18.3§ (8.3) (n=34) | 21.2§ (8.9) (n=24) |
|
| Renal Clearance L/h | 3.1 (0.69) (n=8) |
|||
| Metabolism | Omadacycline is not metabolized | |||
| Excretion (%dose) | Urine | 27 (3.5) (n=8) | 14.4¶ (2.3) (n=6) | ND |
| Feces | ND | 81.1¶ (2.3) (n=6) | ND | |
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
Hyperpigmentation of the thyroid has been produced by members of the tetracycline class in the following species: in rats by omadacycline, oxytetracycline, doxycycline, tetracycline PO4, and methacycline; in minipigs by doxycycline, minocycline, tetracycline PO4, and methacycline; in dogs by doxycycline and minocycline; in monkeys by omadacycline and minocycline.
Minocycline, tetracycline PO4, methacycline, doxycycline, tetracycline base, oxytetracycline HCl, and tetracycline HCl were goitrogenic in rats fed a low iodine diet. This goitrogenic effect was accompanied by high radioactive iodine uptake. Administration of minocycline also produced a large goiter with high radioiodine uptake in rats fed a relatively high iodine diet.
Treatment of various animal species with this class of drugs has also resulted in the induction of thyroid hyperplasia in the following: in rats and dogs (minocycline); in chickens (chlortetracycline); and in rats and mice (oxytetracycline). Adrenal gland hyperplasia has been observed in goats and rats treated with oxytetracycline.
14. Clinical Studies
14.1 Community-Acquired Bacterial Pneumonia
A total of 774 adults with CABP were randomized in a multinational, double-blind, double-dummy trial (Trial 1, NCT #02531438) comparing NUZYRA to moxifloxacin. NUZYRA was administered 100-mg intravenously every 12 hours for two doses on Day 1, followed by 100-mg intravenously daily, or 300-mg orally, daily. Moxifloxacin 400-mg was administered intravenously or orally daily. Total treatment duration was 7-14 days. All enrolled patients were expected to require a minimum of at least 3 days of intravenous treatment. Efficacy and safety of an oral loading dose was not evaluated in CABP.
A total of 386 patients were randomized to NUZYRA and 388 patients were randomized to moxifloxacin. Patient demographic and baseline characteristics were balanced between the treatment groups. Patients were predominantly male (55%) and white (92%). Approximately 60% of patients in each group belonged to PORT Risk Class III, 26% were PORT Risk Class IV and 14.5% were PORT Risk Class II. The median age was 62 years, mean BMI was 27.34 kg/m2, and approximately 47% of NUZYRA treated patients had CrCl <90 ml/min. Among NUZYRA-treated patients, common comorbid conditions included hypertension (49.5%), diabetes mellitus (16.3%), chronic lung disease (21.2%), atrial fibrillation (10.1%), and coronary artery disease (9.1%). The majority of sites were in Eastern Europe, which accounted for 82% of enrollment; 3 patients were enrolled in the US.
Clinical success at the early clinical response (ECR) timepoint, 72 to 120 hours after the first dose, was defined as survival with improvement in at least two of four symptoms (cough, sputum production, chest pain, dyspnea) without deterioration in any of these four symptoms in the intent to treat population (ITT), which consisted of all randomized patients.
Table 7 presents the clinical success rates at the ECR timepoint (ITT population).
| Endpoint | NUZYRA (%) | Moxifloxacin (%) | Treatment Difference (95% CI*) |
|---|---|---|---|
| * Clinical Success at the early clinical response (ECR) timepoint, 72 to 120 hours after the first dose, was defined as survival with improvement in at least two of four symptoms (cough, sputum production, chest pain, dyspnea) from baseline without deterioration in any of these symptoms, with no receipt of antibacterial treatment either as a rescue for CABP or as a treatment for other infections that may be effective for CABP, and no discontinuation of study treatment due to AE. | |||
|
|||
| Clinical Success | 81.1% | 82.7% | -1.6 (-7.1, 3.8) |
Clinical response was also assessed by the investigator at the post therapy evaluation visit (PTE), 5 to 10 days after last dose of study drug and defined as survival and improvement in signs and symptoms of CABP, based on the clinician's judgment, to the extent that further antibacterial therapy is not necessary. Table 8 presents the results of clinical response at the PTE visit for both the ITT population and the Clinically Evaluable (CE) population, which consisted of all ITT patients who had a diagnosis of CABP, received a minimum number of expected doses of study drug, did not have any protocol deviations that would affect the assessment of efficacy, and had investigator assessment at the PTE visit. Clinical response rates by most common baseline pathogen in the microbiological ITT (micro-ITT) population, defined as all randomized patients with a baseline pathogen are presented in Table 9.
| Endpoint | Population | NUZYRA n/N (%) | Moxifloxacin n/N (%) | Treatment Difference (95% CI†) |
|---|---|---|---|---|
|
||||
| Clinical Success at PTE | ITT | 338/386 (87.6) | 330/388 (85.1) | 2.5 (-2.4, 7.4) |
| Clinical Success at PTE | CE | 316/340 (92.9) | 312/345 (90.4) | 2.5 (-1.7, 6.8) |
| Pathogen | NUZYRA n/N (%) | Moxifloxacin n/N (%) |
|---|---|---|
| Streptococcus pneumoniae | 37/43 (86.0) | 31/34 (91.2) |
| Methicillin-susceptible Staphylococcus aureus (MSSA) | 8/11 (72.7) | 8/10 (80.0) |
| Haemophilus influenzae | 26/32 (81.3) | 16/16 (100) |
| Haemophilus parainfluenzae | 15/18 (83.3) | 13/17 (76.5) |
| Klebsiella pneumoniae | 10/13 (76.9) | 11/13 (84.6) |
| Legionella pneumophila | 27/29 (93.1) | 27/28 (96.4) |
| Mycoplasma pneumoniae | 31/35 (88.6) | 25/29 (86.2) |
| Chlamydophila pneumoniae | 14/15 (93.3) | 13/14 (92.9) |
14.2 Acute Bacterial Skin and Skin Structure Infections
A total of 1390 adults with ABSSSI were randomized in two multicenter, multinational, double-blind, double-dummy trials (Trial 2 NCT #02378480 and Trial 3 NCT #02877927). Both trials compared 7 to 14 days of NUZYRA to linezolid. Patients with cellulitis, major abscess, or wound infection were enrolled in the trials.
In Trial 2, 329 patients were randomized to NUZYRA (100-mg intravenously every 12 hours for 2 doses followed by 100-mg intravenously every 24 hours, with the option to switch to 300-mg orally every 24 hours) and 326 patients were randomized to linezolid (600-mg intravenously every 12 hours, with the option to switch to 600-mg orally every 12 hours). Patients in the trial had the following infections: cellulitis (38%), wound infection (33%) and major abscess (29%). The mean surface area of the infected lesion was 455 cm2 in NUZYRA-treated patients and 498 cm2 in linezolid-treated patients. The mean age of patients was 47 years. Subjects were predominantly male (65%) and white (92%), and mean BMI was 28.1 kg/m2. Among NUZYRA-treated patients, common comorbid conditions included drug abuse (53.9%), hepatitis C (29.1%), hypertension (20.4%), anxiety (19.5%), and depression (15.5%). Trial 2 was conducted globally including approximately 60% of patients enrolled in the United States.
In Trial 3, 368 patients were randomized to NUZYRA (450-mg oral once a day on Days 1 and 2, followed by 300-mg orally once a day) and 367 were randomized to linezolid (600-mg orally every 12 hours). All patients were enrolled in the United States. Patients in the trial had the following infections: wound infections (58%), cellulitis (24%), and major abscess (18%). The mean surface area of the infected lesion was 424 cm2 in NUZYRA-treated patients and 399 cm2 in linezolid-treated patients. The mean age of patients was 44 years. Subjects were predominantly male (63%) and white (91%) and mean BMI was 27.9 kg/m2. The most common comorbid conditions included drug abuse (72.8%), tobacco use (12.0%), and chronic hepatitis C infection (31.5%).
In Trials 2 and 3, approximately 12% of NUZYRA treated patients had CrCl <90 ml/min.
In both trials, efficacy was determined by the successful early clinical response at 48 to 72 hours after the first dose in the mITT population and was defined as a 20% or greater decrease in lesion size. Table 10 summarizes the clinical response rates in the two trials. The mITT population was defined as all randomized subjects without a sole Gram-negative causative pathogen at screening.
| Study | NUZYRA (%) | Linezolid (%) | Treatment Difference (Two-Sided 95% CI) † |
|---|---|---|---|
|
|||
| Trial 2 | 84.8 | 85.5 | -0.7 (-6.3, 4.9) |
| Trial 3 | 87.3 | 82.2 | +5.1 (-0.2, 10.5) |
Clinical response at the post therapy evaluation (PTE, 7 to 14 days after last dose) visit in the mITT and clinically evaluable (CE) populations was defined as survival after completion of study treatment without receiving any alternative antibacterial therapy other than NUZYRA, without unplanned major surgical intervention, and sufficient resolution of infection such that further antibacterial therapy is not needed (see Table 11). Clinical response rates at PTE by most common pathogen in the microbiological-mITT population, defined as all patients in the mITT population, who had at least 1 Gram- positive causative pathogen identified at baseline are provided in Table 12. The CE population consisted of all mITT patients who had a diagnosis of ABSSSI, received a minimum number of expected doses of study drug, did not have any protocol deviations that would affect the assessment of efficacy, and had investigator assessment at the PTE Visit.
| Study | Population | NUZYRA n/N (%) | Linezolid n/N (%) | Treatment Difference (Two-Sided 95% CI) * |
|---|---|---|---|---|
|
||||
| Trial 2 | mITT | 272/316 (86.1) | 260/311 (83.6) | +2.5 (-3.2, 8.2) |
| CE | 259/269 (96.3) | 243/260 (93.5) | +2.8 (-1.0, 6.9) | |
| Trial 3 | mITT | 296/353 (83.9) | 284/353 (80.5) | +3.4 (-2.3, 9.1) |
| CE | 272/278 (97.8) | 272/285 (95.4) | +2.4 (-0.6, 5.8) | |
| Pathogen | NUZYRA n/N (%) | Linezolid n/N (%) |
|---|---|---|
| Staphylococcus aureus | 305/369 (82.7) | 306/378 (81.0) |
| Methicillin-susceptible Staphylococcus aureus (MSSA) | 164/201 (81.6) | 181/226 (80.1) |
| Methicillin-resistant Staphylococcus aureus (MRSA) | 146/173 (84.4) | 128/157 (81.5) |
| Staphylococcus lugdunensis | 10/11 (90.9) | 2/3 (66.7) |
| Streptococcus anginosus group | 84/104 (80.8) | 59/82 (72.0) |
| Streptococcus pyogenes | 28/40 (70.0) | 25/34 (73.5) |
| Enterococcus faecalis | 17/18 (94.4) | 21/25 (84.0) |
| Enterobacter cloacae | 11/14 (78.6) | 9/11 (81.8) |
| Klebsiella pneumoniae | 8/11 (72.7) | 6/11 (54.5) |
| NUZYRA
omadacycline injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NUZYRA
omadacycline tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Paratek Pharmaceuticals, Inc. (076333934) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CIPAN | 449040773 | API MANUFACTURE(71715-001, 71715-002) , ANALYSIS(71715-001, 71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Carbogen Amcis AG (Carbogen) | 481385565 | API MANUFACTURE(71715-001, 71715-002) , ANALYSIS(71715-001, 71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Carbogen Amcis AG (Carbogen NE) | 480029695 | ANALYSIS(71715-001, 71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Conframa France (Confarma) | 492738125 | ANALYSIS(71715-001, 71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Solvias AG (Solvias) | 480739627 | ANALYSIS(71715-001, 71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Finorga (Novasep) | 276669694 | API MANUFACTURE(71715-001, 71715-002) , ANALYSIS(71715-001, 71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Alcami Carolinas Corporation (Alcami) | 831351445 | ANALYSIS(71715-001, 71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Almac Pharma Services Limited (Almac) | 233170864 | MANUFACTURE(71715-002) , ANALYSIS(71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Italia S.p.A. (Patheon) | 434078638 | MANUFACTURE(71715-001) , ANALYSIS(71715-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Almac Pharma Services LLC (Almac Audubon) | 078607239 | PACK(71715-001, 71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Corporation (Sharp) | 143696495 | PACK(71715-001, 71715-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sharp Corporation (Sharp) | 002346625 | PACK(71715-002) | |