Drug Detail:Omlonti (Omidenepag isopropyl)
Drug Class: Ophthalmic glaucoma agents
Highlights of Prescribing Information
OMLONTI ® (omidenepag isopropyl ophthalmic solution) 0.002%, for topical ophthalmic use
Initial U.S. Approval: 2022
Indications and Usage for Omlonti Eye Drops
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, is a relatively selective prostaglandin E2 (EP2) receptor agonist, indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. ( 1)
Omlonti Eye Drops Dosage and Administration
The recommended dosage is one drop in the affected eye(s) once daily in the evening. ( 2.1)
Dosage Forms and Strengths
Ophthalmic solution containing 0.002% (0.02 mg/mL) of omidenepag isopropyl. ( 3)
Contraindications
None ( 4)
Warnings and Precautions
- Pigmentation ( 5.1)
- Eyelash changes ( 5.2)
- Ocular Inflammation ( 5.3)
- Macular Edema ( 5.4)
Adverse Reactions/Side Effects
The most common adverse reactions with incidence ≥ 1% are conjunctival hyperemia (9%), photophobia (5%), vision blurred (4%), dry eye (3%), instillation site pain (3%), eye pain (2%), ocular hyperemia (2%), punctate keratitis (2%), headache (2%), eye irritation (1%), and visual impairment (1%). ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Santen at 1-855-7-SANTEN (855-772-6836) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2022
Full Prescribing Information
1. Indications and Usage for Omlonti Eye Drops
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
2. Omlonti Eye Drops Dosage and Administration
2.1 Recommended Dosage
The recommended dosage is one drop in the affected eye(s) once daily in the evening.
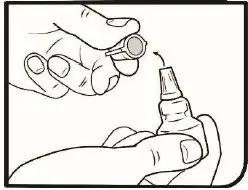
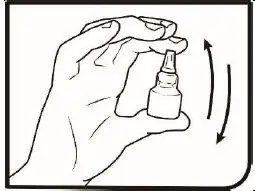
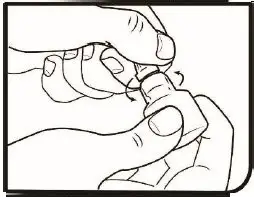
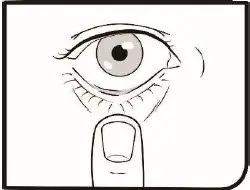
2.2 Administration Instructions
Gently shake the bottle prior to administration. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart. Contact lenses should be removed prior to the administration of Omlonti, and may be reinserted 15 minutes after administration [see Patient Counseling Information (17)].
5. Warnings and Precautions
5.1 Pigmentation
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, is a prodrug of omidenepag, a relatively selective EP2 receptor agonist. Pigmentation is expected to increase as long as omidenepag isopropyl ophthalmic solution is administered. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of Omlonti, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes are likely to be reversible in most patients. Patients who receive prostaglandin analogs, including Omlonti, should be informed of the possibility of increased pigmentation, including permanent changes. The long-term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with Omlonti (omidenepag isopropyl ophthalmic solution), 0.002% can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly [see Patient Counseling Information (17)] .
5.2 Eyelash Changes
Omlonti may gradually change eyelashes and vellus hair in the treated eye. These changes include increased length, thickness, and the number of lashes or hairs. Eyelash changes are usually reversible upon discontinuation of treatment.
5.3 Ocular Inflammation
Ocular inflammation has been reported in patients taking Omlonti. Omlonti should be used with caution in patients with active ocular inflammation, including iritis/uveitis.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Pigmentation [see Warnings and Precautions (5.1)]
- Eyelash Changes [see Warnings and Precautions (5.2)]
- Ocular Inflammation [see Warnings and Precautions (5.3)]
- Macular Edema [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to Omlonti in 600 patients for up to 3 months . The most common adverse reactions with incidence ≥ 1% are conjunctival hyperemia (9%), photophobia (5%), vision blurred (4%), dry eye (3%), instillation site pain (3%), eye pain (2%), ocular hyperemia (2%), punctate keratitis (2%), headache (2%), eye irritation (1%), and visual impairment (1%).
8. Use In Specific Populations
11. Omlonti Eye Drops Description
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, contains the prodrug form of the active omidenepag, a relatively selective prostaglandin EP2 receptor agonist with ocular hypotensive activity. Omidenepag isopropyl is a white to light brown crystal or crystalline powder and practically insoluble in water. Omidenepag isopropyl's chemical name is Glycine, N-[6-[[[[4-(1H-pyrazol-1-yl)phenyl]methyl](3-pyridinylsulfonyl)amino]methyl]-2-pyridinyl]-, 1-methylethyl ester and has the following structure:
Structural Formula
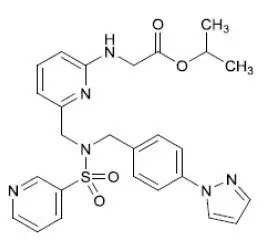
Formula of the free base: C 26H 28N 6O 4S. Molecular weight: 520.61
Omlonti appears as a clear, colorless solution. It is supplied as a sterile, isotonic, buffered aqueous solution of omidenepag isopropyl with a target pH of 5.8 and an osmolality of approximately 285 mOsmol/kg.
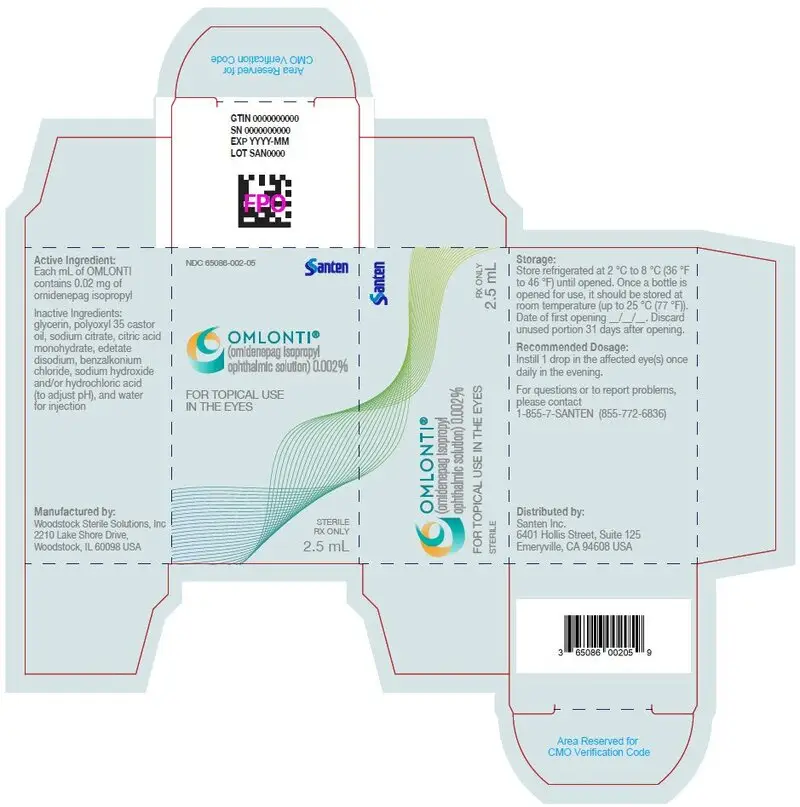
Each mL of Omlonti contains: Active: 0.02 mg of omidenepag isopropyl.
Preservative: 0.005% benzalkonium chloride.
Inactive ingredients: glycerin, polyoxyl 35 castor oil, sodium citrate, citric acid monohydrate, edetate disodium, sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection.
12. Omlonti Eye Drops - Clinical Pharmacology
12.1 Mechanism of Action
Omidenepag is a relatively selective EP2 receptor agonist which decreases intraocular pressure (IOP). The exact mechanism of action is unknown at this time. Elevated IOP represents a major risk factor for glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss.
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
Nasal cavity respiratory epithelium metaplasia was observed in cynomolgus monkeys receiving unilateral topical ocular instillations of omidenepag isopropyl at 0.003% (0.9 mcg/eye) [1.07 fold the MRHOD, based on ocular dose comparison]. In a 13-week monkey study, the 0.9 mcg/eye dose was associated with nasal cavity respiratory epithelial metaplasia, and increased nasal cavity goblet cell respiratory epithelium mucosa. In a 39-week monkey study, the 0.9 mcg/eye dose was associated with increased incidence and severity of nasal cavity respiratory epithelium metaplasia. These findings were present in both the treated side and untreated contralateral side, and they were also observed in the vehicle group [see Adverse Reactions (6)] .
14. Clinical Studies
Omlonti was evaluated in three randomized and controlled clinical trials in subjects with open-angle glaucoma or ocular hypertension with average baseline IOP of 24-26 mm Hg. The double-masked treatment duration was 3 months in all 3 studies. The third study included a 9-month open-label treatment period following the 3-month double-masked treatment period.
In the three studies, IOP reductions were observed for all treatment arms. In the Omlonti arm, the reduction in IOP ranged from 5-7 mm Hg across all three studies. The corresponding reductions for the timolol and latanoprost arms were 5-7 mm Hg and 6-8 mm Hg, respectively.
16. How is Omlonti Eye Drops supplied
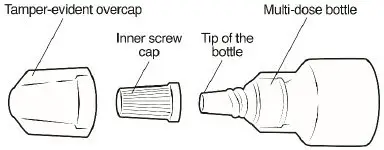
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, is supplied as a 2.5 mL sterile solution in 5 mL white low density polyethylene bottles with linear low density polyethylene dropper tips, high density polyethylene screw caps and tamper-evident low density polyethylene overcaps.
NDC 65086-002-05
| OMLONTI
omidenepag isopropyl solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Santen Incorporated (869321331) |