Drug Detail:Orgovyx (Relugolix [ rel-ue-goe-lix ])
Drug Class: Gonadotropin-releasing hormone antagonists
Highlights of Prescribing Information
ORGOVYX (relugolix) tablets, for oral use
Initial U.S. Approval: 2020
Recent Major Changes
| Contraindications, Hypersensitivity (4). | 03/2023 |
| Warnings and Precautions, Hypersensitivity (5.2). | 03/2023 |
Indications and Usage for Orgovyx
ORGOVYX is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the treatment of adult patients with advanced prostate cancer (1).
Orgovyx Dosage and Administration
- Recommended Dosage: A loading dose of 360 mg on the first day of treatment followed by 120 mg taken orally once daily, at approximately the same time each day (2.1).
- ORGOVYX can be taken with or without food (2.1, 12.3). Instruct patients to swallow tablets whole and not to crush or chew tablets (2.1).
Dosage Forms and Strengths
- Tablets: 120 mg (3).
Contraindications
Known severe hypersensitivity to relugolix or to any of the product components(4).
Warnings and Precautions
- QT/QTc Interval Prolongation: Androgen deprivation therapy may prolong the QT interval (5.1).
- Hypersensitivity: ORGOVYX can cause hypersensitivity reactions, including angioedema. Withhold ORGOVYX in patients who experience symptoms of hypersensitivity. Discontinue ORGOVYX for severe hypersensitivity reactions and manage as clinically indicated (5.2).
- Embryo-Fetal Toxicity: ORGOVYX can cause fetal harm. Advise males with female partners of reproductive potential to use effective contraception (5.3, 8.1, 8.3).
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 10%) and laboratory abnormalities (≥ 15%) were hot flush, glucose increased, triglycerides increased, musculoskeletal pain, hemoglobin decreased, alanine aminotransferase (ALT) increased, fatigue, aspartate aminotransferase (AST) increased, constipation, and diarrhea (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Myovant Sciences, Inc., at 1-833-MYOVANT (1-833-696-8268) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
P-gp Inhibitors: Avoid co-administration. If unavoidable, take ORGOVYX first, separate dosing by at least 6 hours, and monitor patients more frequently for adverse reactions (2.2, 7.1).
Combined P-gp and Strong CYP3A Inducers: Avoid co-administration. If unavoidable, increase the ORGOVYX dose to 240 mg once daily (2.3, 7.1).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
Full Prescribing Information
1. Indications and Usage for Orgovyx
ORGOVYX is indicated for the treatment of adult patients with advanced prostate cancer.
2. Orgovyx Dosage and Administration
2.1 Recommended Dosage
Initiate treatment of ORGOVYX with a loading dose of 360 mg on the first day and continue treatment with a 120 mg dose taken orally once daily at approximately the same time each day.
ORGOVYX can be taken with or without food [see Clinical Pharmacology (12.3)]. Instruct patients to swallow tablets whole and not to crush or chew tablets.
Advise patients to take a missed dose of ORGOVYX as soon as they remember. If the dose was missed by more than 12 hours, patients should not take the missed dose and resume with the next scheduled dose.
If treatment with ORGOVYX is interrupted for greater than 7 days, restart ORGOVYX with a loading dose of 360 mg on the first day, and continue with a dose of 120 mg once daily.
In patients treated with GnRH receptor agonists and antagonists for prostate cancer, treatment is usually continued upon development of nonmetastatic or metastatic castration-resistant prostate cancer.
2.2 Dose Modification for Use with P-gp Inhibitors
Avoid co-administration of ORGOVYX with oral P-gp inhibitors. If co-administration is unavoidable, take ORGOVYX first and separate dosing by at least 6 hours [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Treatment with ORGOVYX may be interrupted for up to two weeks if a short course of treatment with a P-gp inhibitor is required.
2.3 Dose Modification for Use with Combined P-gp and Strong CYP3A Inducers
Avoid co-administration of ORGOVYX with combined P-gp and strong CYP3A inducers. If co-administration is unavoidable, increase the ORGOVYX dose to 240 mg once daily. After discontinuation of the combined P-gp and strong CYP3A inducer, resume the recommended ORGOVYX dose of 120 mg once daily [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
Tablets: 120 mg, light red, almond-shaped, film-coated, and debossed with "R" on one side and "120" on the other side.
4. Contraindications
ORGOVYX is contraindicated in patients with severe hypersensitivity to relugolix or to any of the product components.
5. Warnings and Precautions
5.1 QT/QTc Interval Prolongation
Androgen deprivation therapy, such as ORGOVYX may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, or frequent electrolyte abnormalities and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes [see Clinical Pharmacology (12.2)].
5.2 Hypersensitivity Reactions
ORGOVYX is contraindicated in patients with severe hypersensitivity to relugolix or any of the product components [see Contraindications (4)]. Hypersensitivity reactions, including pharyngeal edema and other serious cases of angioedema, have been reported in postmarketing in patients treated with ORGOVYX.
In the HERO study, patients treated with relugolix reported angioedema (0.2%) [see Clinical Trials Experience (6.1)].
Advise patients who experience any symptoms of hypersensitivity to temporarily discontinue ORGOVYX and promptly seek medical care.
Discontinue ORGOVYX for severe hypersensitivity reactions and manage as clinically indicated.
5.3 Embryo-Fetal Toxicity
The safety and efficacy of ORGOVYX have not been established in females. Based on findings in animals and mechanism of action, ORGOVYX can cause fetal harm and loss of pregnancy when administered to a pregnant female. In an animal reproduction study, oral administration of relugolix to pregnant rabbits during the period of organogenesis caused embryo-fetal lethality at maternal exposures that were 0.3 times the human exposure at the recommended dose of 120 mg daily based on area under the curve (AUC). Advise males with female partners of reproductive potential to use effective contraception during treatment and for 2 weeks after the last dose of ORGOVYX [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
5.4 Laboratory Testing
Therapy with ORGOVYX results in suppression of the pituitary gonadal system. Results of diagnostic tests of the pituitary gonadotropic and gonadal functions conducted during and after ORGOVYX may be affected. The therapeutic effect of ORGOVYX should be monitored by measuring serum concentrations of prostate specific antigen (PSA) periodically. If PSA increases, serum concentrations of testosterone should be measured.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- QT/QTc Interval Prolongation [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
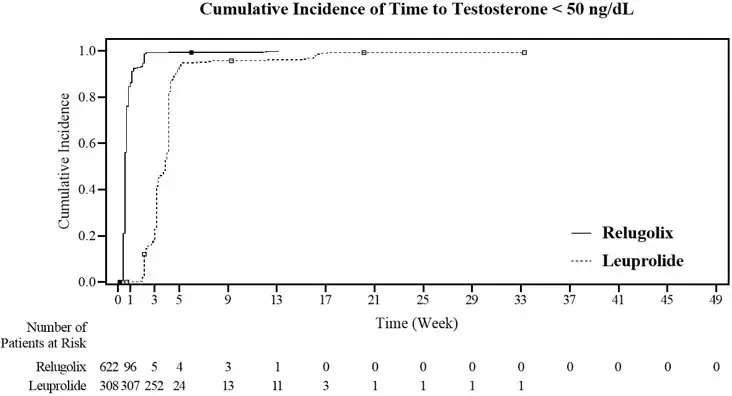
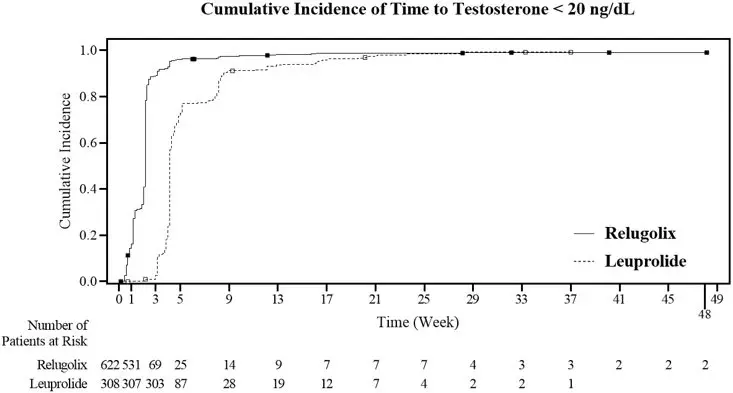
The safety of ORGOVYX was evaluated in HERO, a randomized (2:1), open-label, clinical study in patients with advanced prostate cancer [see Clinical Studies (14)]. Patients received orally administered ORGOVYX as a loading dose of 360 mg on the first day followed by 120 mg taken orally once daily (n = 622) or received leuprolide acetate administered by depot injection at doses of 22.5 mg (n = 264) or 11.25 mg (n = 44) per local guidelines every 12 weeks (n = 308). Leuprolide acetate 11.25 mg is a dosage regimen that is not recommended for this indication in the US. Among patients who received ORGOVYX, 91% were exposed for at least 48 weeks. Ninety-nine (16%) patients received concomitant radiotherapy and 17 (3%) patients received concomitant enzalutamide with ORGOVYX.
Serious adverse reactions occurred in 12% of patients receiving ORGOVYX. Serious adverse reactions in ≥ 0.5% of patients included myocardial infarction (0.8%), acute kidney injury (0.6%), arrhythmia (0.6%), hemorrhage (0.6%), and urinary tract infection (0.5%). Fatal adverse reactions occurred in 0.8% of patients receiving ORGOVYX including metastatic lung cancer (0.3%), myocardial infarction (0.3%), and acute kidney injury (0.2%). Fatal and non-fatal myocardial infarction and stroke were reported in 2.7% of patients receiving ORGOVYX.
Permanent discontinuation of ORGOVYX due to an adverse reaction occurred in 3.5% of patients. Adverse reactions which resulted in permanent discontinuation of ORGOVYX in ≥ 0.3 % of patients included atrioventricular block (0.3%), cardiac failure (0.3%), hemorrhage (0.3%), increased transaminases (0.3%), abdominal pain (0.3%), and pneumonia (0.3%).
Dosage interruptions of ORGOVYX due to an adverse reaction occurred in 2.7% of patients. Adverse reactions which required dosage interruption in ≥ 0.3% of patients included fracture (0.3%).
The most common adverse reactions (≥ 10%) and laboratory abnormalities (≥ 15%), were hot flush (54%), glucose increased (44%), triglycerides increased (35%), musculoskeletal pain (30%), hemoglobin decreased (28%), alanine aminotransferase increased (ALT) (27%), fatigue (26%), aspartate aminotransferase increased (AST) (18%), constipation (12%), and diarrhea (12%).
Table 1 summarizes the adverse reactions in HERO.
| Adverse Reaction | ORGOVYX N = 622 | Leuprolide Acetate N = 308 |
||
|---|---|---|---|---|
| All Grades (%) | Grade 3-4 (%) | All Grades (%) | Grade 3-4 (%) |
|
|
||||
| Vascular disorders | ||||
| Hot flush | 54 | 0.6 | 52 | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Musculoskeletal pain* | 30 | 1.1 | 29 | 1.6 |
| General | ||||
| Fatigue† | 26 | 0.3 | 24 | 0 |
| Gastrointestinal disorders | ||||
| Diarrhea‡ | 12 | 0.2 | 7 | 0 |
| Constipation | 12 | 0 | 10 | 0 |
Clinically relevant adverse reactions in < 10% of patients who received ORGOVYX included increased weight, insomnia, gynecomastia, hyperhidrosis, depression, decreased libido, and angioedema.
Table 2 summarizes the laboratory abnormalities in HERO.
| ORGOVYX* | Leuprolide Acetate* | |||
|---|---|---|---|---|
| Laboratory Test | All Grades (%) | Grade 3-4 (%) | All Grades (%) | Grade 3-4 (%) |
|
||||
| Chemistry | ||||
| Glucose increased | 44 | 2.9 | 54 | 6 |
| Triglycerides increased | 35 | 2 | 36 | 0.7 |
| ALT increased | 27 | 0.3 | 28 | 0 |
| AST increased | 18 | 0 | 19 | 0.3 |
| Hematology | ||||
| Hemoglobin decreased | 28 | 0.5 | 29 | 0.7 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ORGOVYX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: hypersensitivity, including angioedema and urticaria.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and efficacy of ORGOVYX in pediatric patients have not been established.
8.5 Geriatric Use
Of the 622 patients who received ORGOVYX in the HERO study, 81% were 65 years of age or older, while 35% were 75 years of age or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. There was no clinically relevant impact of age on the pharmacokinetics of ORGOVYX or testosterone response based on population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses in men 45 to 91 years of age.
11. Orgovyx Description
Relugolix is a nonpeptide small molecule, GnRH receptor antagonist. The chemical name is N-(4-{1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl}phenyl)-N'-methoxyurea.
The molecular weight is 623.63 daltons and the molecular formula is C29H27F2N7O5S. The structural formula is:
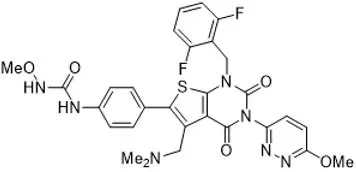
Relugolix is a white to off-white to slightly yellow solid with a solubility of 0.04 mg per mL in water at 25°C.
ORGOVYX is provided as film-coated tablets for oral administration. Each tablet contains 120 mg of relugolix. The inactive ingredients are mannitol, sodium starch glycolate, hydroxypropyl cellulose, magnesium stearate, hypromellose, titanium dioxide, ferric oxide red, and carnauba wax.
12. Orgovyx - Clinical Pharmacology
12.1 Mechanism of Action
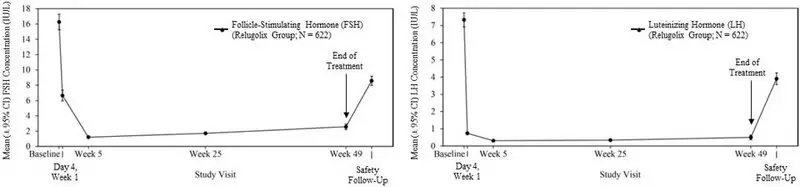
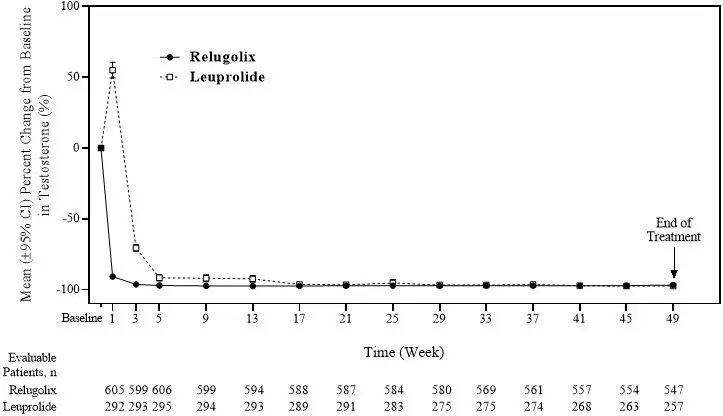
Relugolix is a nonpeptide GnRH receptor antagonist that competitively binds to pituitary GnRH receptors, thereby, reducing the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and consequently testosterone.
12.3 Pharmacokinetics
After administration of single doses ranging from 60 mg to 360 mg (0.17 to 1 times the recommended loading dose), the AUC from time zero extrapolated to infinity (AUC0-inf) and the maximum observed plasma concentration (Cmax) of relugolix increase approximately proportionally with dose. After administration of multiple 20 mg to 180 mg doses of relugolix once daily (0.17 to 1.5 times the recommended once daily dose), the AUCtau of relugolix increases approximately proportionally with dose and the Cmax increases greater than proportionally to dose. After administration of a single 360 mg loading dose in patients, the mean (± standard deviation [± SD]) of AUC0-24 and Cmax of relugolix were 985 (± 742) ng.hr/mL and 215 (± 184) ng/mL, respectively. After administration of a 120 mg dose once daily in patients, the mean (± SD) of AUC0-24 and Cmax of relugolix at steady-state were 407 (± 168) ng.hr/mL and 70 (± 65) ng/mL, respectively. The accumulation of relugolix upon once daily administration is approximately 2-fold.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in mice at oral relugolix doses up to 100 mg/kg/day and in rats at doses up to 600 mg/kg/day. Relugolix was not carcinogenic in mice or rats at exposures up to approximately 75 or 224 times, respectively, the human exposure at the recommended dose of 120 mg daily based on AUC.
Relugolix was not mutagenic in the in vitro bacterial reverse mutation (Ames) assay or clastogenic in the in vitro chromosomal aberration assay in Chinese hamster lung cells or the in vivo rat bone marrow micronucleus assay.
In human GnRH-receptor knock-in male mice, oral administration of relugolix decreased prostate and seminal vesicle weights at doses ≥ 3 mg/kg twice daily for 28 days. The effects of relugolix were reversible, except for testis weight, which did not fully recover within 28 days after drug withdrawal. In a 39-week repeat-dose toxicity study in monkeys, there were no significant effects on male reproductive organs at oral relugolix doses up to 50 mg/kg/day (approximately 53 times the human exposure at the recommended dose of 120 mg daily based on AUC).
13.2 Animal Toxicology and/or Pharmacology
Phospholipidosis (intracellular phospholipid accumulation) was observed in multiple organs and tissues (e.g., liver, pancreas, spleen, kidney, lymph nodes, lung, bone marrow, gastrointestinal tract or testes) after repeated oral administration of relugolix in rats and monkeys. In a rat 26-week toxicity study, phospholipidosis was observed at doses ≥ 100 mg/kg (approximately 18 times the human exposure at the recommended dose based on AUC). In a monkey 39-week toxicity study, this effect was observed at doses ≥ 1.5 mg/kg (approximately 0.6 times the human exposure at the recommended dose based on AUC) and demonstrated evidence of reversibility after cessation of treatment. The significance of this finding in humans is unknown.
| ORGOVYX
relugolix tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Myovant Sciences, Inc. (080453011) |
| Registrant - Myovant Sciences GmbH (480146015) |