Drug Detail:Parnate (Tranylcypromine [ tran-il-sip-roe-meen ])
Drug Class: Monoamine oxidase inhibitors
Highlights of Prescribing Information
Parnate® (tranylcypromine) tablets, for oral use
Initial U.S. Approval: 1961
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS AND HYPERTENSIVE CRISIS WITH SIGNIFICANT TYRAMINE USE
See full prescribing information for complete boxed warning.
- Increased risk of suicidal thoughts and behavior in pediatric and young adult patients taking antidepressants. Closely monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors. PARNATE is not approved for use in pediatric patients. (5.1, 8.4)
- Excessive consumption of foods or beverages with significant tyramine content or certain drugs can precipitate hypertensive crisis. Monitor blood pressure, allow for medication free intervals, and advise patients to avoid foods and beverages with high tyramine content. (5.2, 7.1, 7.2)
Indications and Usage for Parnate
- PARNATE is a monoamine oxidase inhibitor (MAOI) indicated for the treatment of major depressive disorder (MDD) in adult patients who have not responded adequately to other antidepressants (1)
- PARNATE is not indicated for the initial treatment of MDD due to the potential for serious adverse reactions and drug interactions, and the need for dietary restrictions (1, 4,5,7)
Parnate Dosage and Administration
- Recommended daily dosage is 30 mg in divided doses (2.1)
- If no adequate response, increase dosage in increments of 10 mg per day every 1 to 3 weeks to a maximum dosage of 30 mg twice daily (60 mg per day). Consider more gradual dosage increases in patients at risk for hypotension (2.1)
- Consider discontinuing PARNATE therapy gradually because of the risk for withdrawal effects (2.3,5.8,9.3)
- Switching from or to other MAOIs or other antidepressants: See full prescribing information for instructions (2.2,7.1)
Dosage Forms and Strengths
Tablets: 10 mg (3)
Contraindications
- Concomitant use or use in rapid succession with other MAOIs; selective serotonin reuptake inhibitors; serotonin and norepinephrine reuptake inhibitors; tricyclic antidepressants; sympathomimetic drugs; and numerous other drugs. See Full Prescribing Information for the full list of contraindicated products (4.1, 7.1)
- Pheochromocytoma, other catecholamine-releasing paraganglioma (4.2)
Warnings and Precautions
- Activation of Mania/Hypomania: May be precipitated by antidepressant treatment in patients with bipolar disorder.Screen patients prior to treatment (5.4)
- Hypotension (including syncope): Monitor patients and adjust PARNATE dosage or concomitant medication as necessary (5.5)
- Hypotension and Hypertension during Anesthesia and Perioperative Care: If possible, discontinue PARNATE prior to elective surgery (5.6)
- Hepatitis and Elevated Liver Enzymes: Monitor accordingly (5.10)
Adverse Reactions/Side Effects
Most common adverse reactions (>10%) were dry mouth, dizziness, insomnia, sedation, headache, overexcitement, constipation, blurred vision, and tremor (6)
To report SUSPECTED ADVERSE REACTIONS, contact Concordia Pharmaceuticals at 1-877-370-1142 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
See Full Prescribing Information for a list of products, foods and beverages that can interact with PARNATE. (7)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2021
Related/similar drugs
Rexulti, Trintellix, sertraline, trazodone, Lexapro, citalopram, ZoloftFull Prescribing Information
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS and HYPERTENSIVE CRISIS WITH SIGNIFICANT TYRAMINE USE
Suicidal Thoughts and Behaviors
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. PARNATE is not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
Hypertensive Crisis with Significant Tyramine Use
Excessive consumption of foods or beverages with significant tyramine content or the use of certain drugs with PARNATE or after PARNATE discontinuation can precipitate hypertensive crisis. Monitor blood pressure and allow for medication-free intervals between administration of PARNATE and interacting drugs. Instruct patients to avoid ingestion of foods and beverages with high tyramine content [see Warnings and Precautions (5.2) and Drug Interactions(7.1, 7.2)].
1. Indications and Usage for Parnate
PARNATE is indicated for the treatment of major depressive disorder (MDD) in adult patients who have not responded adequately to other antidepressants. PARNATE is not indicated for the initial treatment of MDD due to the potential for serious adverse reactions and drug interactions, and the need for dietary restrictions [see Contraindications (4), Warnings and Precautions (5), and Drug Interactions (7)].
2. Parnate Dosage and Administration
2.1 Recommended Dosage
PARNATE tablets are for oral use. The recommended dosage is 30 mg per day (in divided doses). If patients do not have an adequate response, increase the dosage in increments of 10 mg per day every 1 to 3 weeks to a maximum 30 mg twice daily (60 mg per day). Dosage increases should be made more gradually in patients at risk for hypotension (e.g., geriatric patients) [see Warnings and Precautions (5.5)].
2.2 Switching to or from Other Antidepressants
Switching from Contraindicated Antidepressants to PARNATE
After stopping treatment with contraindicated antidepressants, a time period of 4 to 5 half-lives of the other antidepressant or any active metabolite should elapse before starting treatment with PARNATE. After stopping treatment with an MAO inhibitor antidepressant, a time period of at least one week or 4 to 5 half-lives of the other MAO inhibitor (whichever is longer) should elapse before starting treatment with PARNATE to reduce the risk of additive effects [see Contraindications (4.1) and Drug Interactions (7.1)].
Switching from PARNATE to Other MAOIs or Contraindicated Antidepressants
After stopping PARNATE treatment, at least one week should elapse before starting another MAOI (intended to treat MDD) or other contraindicated antidepressants. Refer to the prescribing information of the subsequently used drug for product-specific advice on a medication-free interval [see Contraindications (4.1) and Drug Interactions (7.1)].
2.3 Discontinuing Treatment
Withdrawal effects, including delirium, have been reported with abrupt discontinuation of PARNATE therapy. Higher daily doses and longer duration of use appear to be associated with a higher risk of withdrawal effects. Consider discontinuing PARNATE therapy by slow, gradual dosage reduction [see Warnings and Precautions (5.8) and Drug Abuse and Dependence (9.3)].
3. Dosage Forms and Strengths
Tablets containing tranylcypromine sulfate equivalent to 10 mg tranylcypromine are round, rose‑red, film‑coated, and debossed on one side with “PARNATE” and “SB.”
4. Contraindications
4.1 Combination with Certain Drugs
Concomitant use of PARNATE or use in rapid succession with the products in Table 1 is contraindicated. Such use may cause severe or life-threatening reactions such as hypertensive crises or serotonin syndrome [see Drug Interactions (7.1)]. Medication-free periods between administration of PARNATE and contraindicated agents are recommended [see Dosage and Administration (2.2)and Drug Interactions (7.1)].
Table 1: Products Contraindicated with the Use of PARNATE
| Drug Classes | ||
| Non-selective H1 receptor antagonists | ||
Antidepressants including but not limited to:
|
||
| Amphetamines and methylphenidates and derivatives | ||
| Sympathomimetic products (e.g., cold, hay fever or weight reducing products that contain vasoconstrictors such as pseudoephedrine, phenylephrine, and ephedrine; or dietary supplements that contain sympathomimetics) | ||
| Triptans | ||
| Individual Drugs (not included in the above classes) | ||
| buspirone | levodopa | s-adenosyl-L-methionine (SAM-e) |
| carbamazepine | meperidine | tapentadol |
| cyclobenzaprine | methyldopa | tetrabenazine |
| dextromethorphan | milnacipran | tryptophan |
| dopamine | rasagiline | |
| hydroxytryptophan | reserpine | |
5. Warnings and Precautions
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 2.
Table 2: Risk Differences of the Number of Patients of Suicidal Thoughts and Behavior in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients
| Age Range
|
Drug-Placebo Difference in Number of Patients of Suicidal Thoughts or Behaviors per 1000 Patients Treated |
| | Increases Compared to Placebo
|
| <18 years old | 14 additional patients |
| 18-24 years old | 5 additional patients |
| | Decreases Compared to Placebo
|
| 25-64 years old | 1 fewer patient |
| ≥65 years old | 6 fewer patients |
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for any indication for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing PARNATE, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 Hypertensive Crisis and Hypertension
Hypertensive Crisis
MAOIs, including PARNATE, have been associated with hypertensive crises caused by the ingestion of foods or beverages with a high concentration of tyramine. In addition, hypertensive reactions and crises may occur with concomitant use of other drugs [see Drug Interactions (7.1)]. Patients with hyperthyroidism may be at greater risk of hypertensive crisis.
Signs, Symptoms, and Complications of Hypertensive Crisis: In some patients a hypertensive crisis constitutes a hypertensive emergency, which requires immediate attention to prevent serious complications or fatal outcome. These emergencies are characterized by severe hypertension (e.g., with a blood pressure of more than 180/120 mm Hg) and evidence of organ dysfunction. Symptoms may include occipital headache (which may radiate frontally), palpitations, neck stiffness or soreness, nausea or vomiting, sweating (sometimes with fever or cold, clammy skin), dilated pupils, photophobia, shortness of breath, or confusion. Either tachycardia or bradycardia may be present and may be associated with constricting chest pain. Seizures may also occur. Intracranial bleeding, sometimes fatal, has been reported in association with the increase in blood pressure.
Strategies to Reduce the Risk of Hypertensive Crisis: Instruct patients to avoid foods and beverages with high tyramine content while being treated with PARNATE and for 2 weeks after stopping PARNATE [see Drug Interactions (7.2)]. Careful evaluation of the benefits and risks of PARNATE therapy is necessary in patients with:
- Hypertension or confirmed or suspected cerebrovascular or cardiovascular disorders that constitute an increased risk for complications from severe hypertension, and
- A history of headaches that can mask the occurrence of headaches as prodromal of a hypertensive crisis.
In all patients taking PARNATE, monitor blood pressure closely to detect evidence of increased blood pressure. Full reliance should not be placed on blood pressure readings. The patient should also be observed for other signs and symptoms of hypertensive crisis.
Treatment of Hypertensive Crisis: Therapy should be interrupted with symptoms that may be prodromal or a manifestation of a hypertensive crisis, such as palpitations or headaches, and patients should be evaluated immediately. Discontinue PARNATE, other drugs, foods or beverages suspected to contribute to the hypertensive crisis immediately [see Drug Interactions (7.1, 7.2)].
Patients with severe elevations in blood pressure (e.g., more than 180/120 mm Hg) with evidence of organ dysfunction require immediate blood pressure reduction. Fever should be managed by means of external cooling. However, additional measures to control the causes of hyperthermia (psychomotor agitation, increased neuromuscular activity, persistent seizures) may be required.
Hypertension
Clinically significant increases in blood pressure have also been reported after the administration of MAOIs, including PARNATE, in patients not ingesting tyramine-rich foods or beverages. Assess blood pressure before prescribing PARNATE and closely monitor blood pressure in all patients taking PARNATE.
5.3 Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with MAOIs when used concomitantly with other serotonergic drugs. Such drugs include SSRIs, SNRIs, tricyclic antidepressants, triptans, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John’s wort, S-adenosyl-L-methionine (SAM-e), and other MAOIs used to treat nonpsychiatric disorders (such as linezolid or intravenous methylene blue).
Manifestations of the serotonin syndrome may include mental status changes (e.g., agitation, hallucinations, delirium, coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia; with possible rapid fluctuations of vital signs), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyper-reflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Fatal outcome of serotonin syndrome has been reported, including in patients who had been treated with PARNATE. In some cases of an interaction between PARNATE and SSRIs or SNRIs, the features of the syndrome resembled neuroleptic malignant syndrome.
The concomitant use, or use in rapid succession, of PARNATE with other serotonergic drugs is contraindicated. However, there may be circumstances when treatment with other serotonergic substances (such as linezolid or intravenous methylene blue) is necessary and cannot be delayed. In such cases, PARNATE must be discontinued as soon as possible before initiating treatment with the other agent.
Treatment with PARNATE and any concomitant serotonergic agents should be discontinued immediately if the above events occur, and supportive symptomatic treatment should be initiated.
5.4 Activation of Mania/Hypomania
In patients with bipolar disorder, treating a depressive episode with PARNATE or another antidepressant may precipitate a mixed/manic episode. Prior to initiating treatment with PARNATE, screen patients for any personal or family history of bipolar disorder, mania, or hypomania.
5.5 Hypotension
Hypotension, including postural hypotension, has been observed during therapy with PARNATE. At doses above 30 mg daily, postural hypotension is a major adverse reaction and may result in syncope. Symptoms of postural hypotension are seen most commonly, but not exclusively, in patients with pre-existing hypertension. Blood pressure usually returns rapidly to pretreatment levels upon discontinuation of PARNATE.
Dosage increases should be made more gradually in patients with a tendency toward hypotension and/or postural hypotension (e.g., elderly patients) [see Dosage and Administration (2.2) and Use in Specific Populations (8.5)]. Such patients should be closely observed for postural changes in blood pressure throughout treatment. Also, when PARNATE is used concomitantly with other agents known to cause hypotension, the possibility of additive hypotensive effects should be considered [see Drug Interactions (7.1)]. Postural hypotension may be relieved by having patients lie down until blood pressure returns to normal.
5.6 Hypotension and Hypertension during Anesthesia and Perioperative Care
It is recommended that PARNATE be discontinued at least 10 days prior to elective surgery. If this is not possible, for general anesthesia, regional and local anesthesia, and perioperative care avoid the use of agents that are contraindicated for concomitant use with PARNATE. Carefully consider the risk of agents and techniques that increase the risk for hypotension (e.g., epidural or spinal anesthesia) or other adverse reactions to PARNATE (e.g., hypertension associated with the use of vasoconstrictors in local anesthetics).
5.7 Need for Emergency Treatment with Contraindicated Drugs
If in the absence of therapeutic alternatives emergency treatment with a contraindicated product (e.g., linezolid, intravenous methylene blue, direct-acting sympathomimetic drugs such as epinephrine) becomes necessary and cannot be delayed, discontinue PARNATE as soon as possible before initiating treatment with the other product and monitor closely for adverse reactions [see Drug Interactions (7.1)]
5.8 Discontinuation Syndrome
Abrupt discontinuation or dosage reduction of PARNATE has been associated with the appearance of new symptoms that include dizziness, nausea, headache, irritability, insomnia, diarrhea, anxiety, fatigue, abnormal dreams, and hyperhidrosis. In general, discontinuation events occurred more frequently with longer duration of therapy.
There have been spontaneous reports of adverse reactions occurring upon discontinuation of MAOIs, particularly when abrupt, including dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g. paresthesia, such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. While these reactions are generally self-limiting, there have been reports of prolonged discontinuation symptoms.
Patients should be monitored for these symptoms when discontinuing treatment with PARNATE. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible [see Dosage and Administration (2.3) and Adverse Reactions (6)].
5.9 Risk of Clinically Significant Adverse Reactions due to Persistence of MAO Inhibition after Discontinuation
Although excretion of PARNATE is rapid, inhibition of MAO may persist up to 10 days following discontinuation. This should be taken into account when considering the use of potentially interacting substances or the consumption of tyramine-rich food or beverages [see Drug Interactions (7.2)], or when interpreting adverse reactions observed after discontinuation of PARNATE. Care should be taken to differentiate symptoms of persistent MAO inhibition from withdrawal symptoms [see Drug Abuse and Dependence (9.3)].
5.10 Hepatotoxicity
Hepatitis and elevated aminotransferases have been reported in association with PARNATE administration. Patients should be monitored accordingly. PARNATE should be discontinued in patients who develop signs and symptoms of hepatotoxicity.
Sedation has occurred in PARNATE-treated patients with cirrhosis. Patients with cirrhosis receiving PARNATE should be monitored for possible increased risks of central nervous system adverse reactions, such as excessive drowsiness.
5.11 Seizures
Seizures have been reported with PARNATE withdrawal after abuse, and with overdose. Patients at risk for seizures should be monitored accordingly.
5.12 Hypoglycemia in Diabetic Patients
Some MAOIs have contributed to hypoglycemic episodes in diabetic patients receiving insulin or other blood-glucose-lowering agents. Monitor blood glucose in patients receiving both PARNATE and blood-glucose-lowering agents. A reduction of the dosage of such agents may be necessary [see Drug Interactions (7.1)]
5.13 Aggravation of Coexisting Symptoms of Depression
PARNATE may aggravate coexisting symptoms in depression, such as anxiety and agitation.
5.14 Adverse Effects on the Ability to Drive and Operate Machinery
Some PARNATE adverse reactions (e.g., hypotension, faintness, drowsiness, confusion, disorientation) can impair a patient’s ability to operate machinery or use an automobile. Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that PARNATE therapy does not impair their ability to engage in such activities.
6. Adverse Reactions/Side Effects
The following adverse reactions are described in greater detail in other sections:
- Suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]
- Hypertensive crisis and hypertension [see Warnings and Precautions (5.2)]
- Serotonin syndrome [see Warnings and Precautions (5.3)]
- Activation of mania/hypomania [see Warnings and Precautions (5.4)]
- Hypotension [see Warnings and Precautions (5.5)]
- Hypotension and hypertension during anesthesia and perioperative care [see Warnings and Precautions (5.6)]
- Discontinuation syndrome [ see Warnings and Precautions (5.8)]
- Persistence of MAO inhibition after discontinuation [see Warnings and Precautions (5.9)]
- Hepatotoxicity [see Warnings and Precautions (5.10)]
- Seizures[see Warnings and Precautions (5.11)]
- Hypoglycemia in diabetic patients [see Warnings and Precautions (5.12)]
- Aggravation of coexisting symptoms of depression [see Warnings and Precautions (5.13)]
- Adverse effects on the ability to drive and operate machinery [see Warnings and Precautions (5.14)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Based on clinical trial data, the most common adverse reactions to tranylcypromine were dry mouth, dizziness, insomnia, sedation, and headache (>30%) and overexcitement, constipation, blurred vision, and tremor (>10%).
The following adverse reactions have been identified in clinical trials or during postapproval use of PARNATE:
Blood and lymphatic system disorders: agranulocytosis, leukopenia, thrombocytopenia, anemia
Endocrine disorders: impaired water excretion compatible with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH)
Metabolism and nutrition disorders: significant anorexia, weight gain
Psychiatric disorders: excessive stimulation/overexcitement, manic symptoms/hypomania, agitation, insomnia, anxiety, confusion, disorientation, loss of libido
Nervous system disorders: dizziness, restlessness/akathisia, akinesia, ataxia, myoclonic jerks, tremor, hyper-reflexia, muscle spasm, paresthesia, numbness, memory loss, sedation, drowsiness, dysgeusia, headaches (without blood pressure elevation)
Eye disorders: blurred vision, nystagmus
Ear and labyrinth disorders: tinnitus
Cardiac disorders: tachycardia, palpitations
Vascular disorders: hypertensive crisis, hypertension, hypotension (including postural hypotension with syncope)
Gastrointestinal disorders: diarrhea, constipation, nausea, abdominal pain, dry mouth, fissuring in corner of mouth
Hepatobiliary disorders: hepatitis, elevated aminotransferases
Skin and subcutaneous tissue disorders: localized scleroderma, flare-up of cystic acne, urticaria, rash, alopecia, sweating
Renal and urinary disorders: urinary retention, urinary incontinence, urinary frequency
Reproductive system and breast disorders: impotence, delayed ejaculation
General disorders and administration site conditions: edema, chills, weakness, fatigue/lethargy
7. Drug Interactions
7.1 Clinically Significant Drug Interactions
Tables 3 and 4 lists drug classes and individual products, respectively, with a potential for interaction with PARNATE, describes the predominant observed or anticipated risks, and provides advice on concomitant use. Given serious adverse reactions with multiple agents, patients should avoid taking over-the-counter medications or dietary supplements without prior consultation with a healthcare provider able to provide advice on the potential for interactions.
Time to Start PARNATE after Discontinuation of a Contraindicated Drug
For products that are contraindicated with PARNATE, a time period of 4 to 5 half-lives of the other product or any active metabolite should elapse before starting treatment with PARNATE. After stopping treatment with an MAO inhibitor antidepressant, a time period of at least 1 week or 4 to 5 half-lives of the other MAO inhibitor (whichever is longer) should elapse before starting treatment with PARNATE because of the risk for clinically significant adverse reactions after discontinuation due to persistent MAO inhibition [see Dosage and Administration (2.2), Warnings and Precautions (5.9) ]. This period can be several weeks long (e.g., a minimum of 5 weeks for fluoxetine given fluoxetine's long half-life). Refer to the prescribing information of the contraindicated product for relevant information.
Time to Start Contraindicated Drug after Discontinuation of PARNATE
The potential for interactions persists after discontinuation of PARNATE until MAO activity has sufficiently recovered. Inhibition of MAO may persist up to 10 days following discontinuation [see Warnings and Precautions (5.9)]. After stopping PARNATE, at least 1 week should elapse before starting another MAOI (intended to treat MDD) or other contraindicated antidepressants. Refer to the prescribing information of any agent considered for subsequent use for recommendations on the duration of a waiting period after discontinuation of a MAO inhibitor.
If in the absence of therapeutic alternatives and emergency treatment with a contraindicated drug (e.g., linezolid, intravenous methylene blue, direct-acting sympathomimetic drugs such as epinephrine) becomes necessary and cannot be delayed, discontinue PARNATE as soon as possible before initiating treatment with the other agent, and monitor closely for adverse reactions.
Table 3 Clinically Significant Drug Interactions with Drug Classes*
|
Product |
Clinical Comment on Concomitant Usea |
Predominant Effect/Risk[Hypertensive Reaction (HR)b or Serotonin Syndrome (SS)c] |
|
Agents with blood pressure-reducing effects |
Use with cautiond |
Hypotensione |
|
Non-selective H1 receptor antagonists |
Contraindicateda |
Increased anticholinergic effects |
|
Beta-adrenergic blockers (see also agents or procedures with blood pressure-reducing effects) |
Use with the cautiond |
More pronounced bradycardia, postural hypotensione |
|
Blood glucose-lowering agents |
Dosage reduction of such agents may be necessary. Monitor blood glucose. |
Excessive reduction of blood glucose (additive effect)f |
|
CNS depressant agents (including opioids, alcohol, sedatives, hypnotics) |
Use with cautiond |
Increased CNS depression |
|
Dietary supplements containing sympathomimetics |
Contraindicateda |
|
Antidepressants including but not limited to:
|
Contraindicateda |
SS for all antidepressants For MAOIs, increased MAO inhibition and risk of adverse reactions, SS, and HRg |
|
Amphetamines and methylphenidates and derivatives |
Contraindicateda |
HR |
|
Sympathomimetic drugs** |
Contraindicateda |
HR; Including risk of intracerebral hemorrhage |
|
Triptans |
Contraindicateda |
SS |
* Some drugs in these groups may also be listed in Table 4 below.
** Sympathomimetic drugs include amphetamines as well as cold, hay fever or weight-reducing products that contain vasoconstrictors such as pseudoephedrine, phenylephrine, and ephedrine)
a[See Contraindications (4.1)]; b[See Warnings and Precautions (5.2)]; c[See Warnings and Precautions (5.3)]
d If not otherwise specified in this table, consider avoiding concomitant use (see also information on medication-free intervals, use agent at the lowest appropriate dosage, monitor for effects of the interaction, advise the patient to report potential effects).
e [See Warnings and Precautions (5.5)]; f [See Warnings and Precautions (5.14)]; g[See Overdosage (10.1)]
Table 4: Clinically Significant Drug Interactions with Individual Products*
|
Product |
Clinical Comment on Concomitant Usea |
Predominant Effect/Risk [Hypertensive Reaction (HR)b or Serotonin Syndrome (SS)c] |
|
Altretamine |
Use with cautiond |
Orthostatic hypotensione |
|
Buspirone |
Contraindicateda |
HR |
|
Carbamazepine |
Contraindicateda |
SS |
|
Chlorpromazine |
Use with cautiond |
Hypotensive effectse |
|
Cyclobenzaprine |
Contraindicateda |
SS |
|
Dextromethorphan |
Contraindicateda |
SS; Psychosis, bizarre behavior |
|
Dopamine |
Contraindicateda |
HR |
|
Droperidol |
Use with cautiond |
QT interval prolongation |
|
Entacapone |
Use with cautiond |
HR |
|
Fentanyl |
Use with cautiond |
SS |
|
Hydroxytryptophan |
Contraindicateda |
SS |
|
Levodopa |
Contraindicateda |
HR |
|
Lithium |
Use with cautiond |
SS |
|
Meperidine |
Contraindicateda |
SS |
|
Methadone |
Use with cautiond |
SS |
|
Methyldopa |
Contraindicateda |
HR |
|
Metoclopramide |
Use with cautiond |
HR/SS |
|
Mirtazapine |
Contraindicateda |
SS |
|
Oxcarbazepine |
Use with cautiond because of close structural relationship with tricyclic antidepressants |
SS |
|
Rasagiline |
Contraindicateda |
HR |
|
Reserpine |
Contraindicateda |
HR |
|
S-adenosyl-L-methionine (SAM-e) |
Contraindicateda |
SS |
|
Tapentadol |
Contraindicateda |
HR/SS |
|
Tetrabenazine |
Contraindicateda |
HR |
|
Tolcapone |
Use with cautiond |
HR |
|
Tramadol |
Use with cautiond |
SS; Increased seizure risk |
|
Tryptophan |
Contraindicateda |
SS |
*Some drugs in this table may also belong to groups listed in Table 3 above, and may be associated with additional interactions.
a[See Contraindications (4.1)]; b[See Warnings and Precautions (5.3)]; c[See Warnings and Precautions (5.7)] dIf not otherwise specified in this table, consider avoiding concomitant use (see also information on medication-free intervals , use agent at the lowest appropriate dose, monitor for effects of the interaction, advise the patient to report potential effects, and be prepared to discontinue the agent and treat effects of the interaction
e[See Warnings and Precautions (5.5)]
7.2 Tyramine-Containing Foods and Beverages
PARNATE inhibits intestinal MAO, which is responsible for the catabolism of tyramine in food and beverages. As a result of this inhibition, large amounts of tyramine may enter the systemic circulation and precipitate a sudden elevation in blood pressure or hypertensive crisis [see Warnings and Precautions (5.2)]. Instruct PARNATE-treated patients to avoid foods and beverages with significant tyramine content during treatment with PARNATE or within 2 weeks of stopping treatment (see Table 5 for a list of food and beverages containing significant amounts of tyramine).
Table 5: Foods and Beverages with and without Significant Amounts of Tyramine
| Class of Food or
Beverage | Tyramine-Rich Foods and
Beverages to Avoid | Acceptable Foods and Drinks, Containing No or Little Tyramine
|
| Meat, Poultry, and Fish | Air dried, aged and fermented meats, sausages and salamis (including cacciatore, hard salami and mortadella); pickled herring; and any spoiled or improperly stored meat, poultry, and fish (e.g., foods that have undergone changes in coloration, odor, or become moldy); spoiled or improperly stored animal livers | Fresh meat, poultry, and fish, including fresh processed meats (e.g., lunch meats, hot dogs, breakfast sausage, and cooked sliced ham) |
| Vegetables | Broad bean pods (fava bean pods) | All other vegetables |
| Dairy | Aged cheeses | Processed cheeses, mozzarella, ricotta cheese, cottage cheese, and yogurt |
| Beverages | All varieties of tap beer and beers that have not been pasteurized so as to allow for ongoing fermentation and excessive amounts of caffeine. | Concomitant use of alcohol with PARNATE is not recommended. (Bottled and canned beers and wines contain little or no tyramine.) |
| Other | Concentrated yeast extract (e.g., Marmite), sauerkraut, most soybean products (including soy sauce and tofu), OTC supplements containing tyramine, and chocolate | Brewer’s yeast, baker’s yeast, soy milk, commercial chain restaurant pizzas prepared with cheeses low in tyramine |
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are limited published reports of placental infarction and congenital anomalies in association with use of PARNATE during pregnancy; however, these reports may not adequately inform the presence or absence of drug-associated risk with the use of PARNATE during pregnancy. In the U.S. general population, the background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. Animal embryo-fetal development studies were not conducted with tranylcypromine; however, published animal reproduction studies report placental transfer of tranylcypromine in rats and a dose-dependent decrease in uterine blood flow in pregnant sheep. Advise pregnant women of the potential risk to a fetus.
Clinical Considerations
Labor or Delivery
During labor and delivery, the potential for interactions between PARNATE and drugs or procedures (e.g., epidural anesthesia) should be taken into account in women who have received PARNATE [see Warnings and Precautions (5.6) and Drug Interactions (7.1)].
8.2 Lactation
Risk Summary
Tranylcypromine is present in human milk. There is no available information on the effects of tranylcypromine on milk production. There is no available information on the effects of tranylcypromine on a breastfed child; however, because of the potential for serious adverse reactions in a breastfed infant, advise nursing women to discontinue breastfeeding during treatment with PARNATE
8.4 Pediatric Use
Safety and effectiveness of PARNATE in the pediatric population have not been established. All risks associated with the use of PARNATE, including the risk of suicidal thoughts and behavior, apply to adults and pediatric patients [see Boxed Warning and Warnings and Precautions (5)].
8.5 Geriatric Use
Older patients may be at greater risk of postural hypotension and other serious adverse reactions[see Warnings and Precautions (5)]. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
9. Drug Abuse and Dependence
9.2 Abuse
Abuse of PARNATE has been reported. Some of these patients had a history of previous substance abuse.
The potential for abuse and the increased risk of serious adverse reactions with higher doses should be taken into account when considering the use of PARNATE for patients at increased risk for substance abuse.
9.3 Dependence
Dependence, evidenced by precipitation of withdrawal effects following abrupt discontinuation of PARNATE has been reported. Reported withdrawal effects included delirium (even with low daily doses), restlessness, anxiety, confusion, hallucinations, headache, weakness, diarrhea, and/or rapid relapse into depression. Thrombocytopenia and liver enzyme increases have also been observed in association with PARNATE withdrawal from high doses [see Overdosage (10.1)]
Withdrawal effects have appeared within 1 to 3 days of discontinuation and have persisted for several weeks after discontinuation. The use of daily doses greater than recommended and longer duration of use appear to be associated with a higher risk of withdrawal effects.
Monitor for withdrawal effects for at least 1 week after discontinuation. Consider discontinuing PARNATE therapy by slow, gradual dose reduction [see Dosage and Administration (2.3)].
10. Overdosage
10.1 Overdosage Symptoms, Signs, and Laboratory Abnormalities
Overdose of PARNATE can cause the adverse reactions generally associated with PARNATE administration [see Warnings and Precautions (5), Adverse Reactions (6)and Drug Interactions (7.1)]. However, these reactions may be more severe, including fatal reactions. Effects reported with overdosage of PARNATE and/or other MAOIs include:
- Insomnia, restlessness, and anxiety, progressing in severe cases to agitation, mental confusion, and incoherence; delirium; seizures
- Hypotension, dizziness, weakness, and drowsiness, progressing in severe cases to extreme dizziness and shock
- Hypertension with severe headache and other symptoms/complications
- Twitching or myoclonic fibrillation of skeletal muscles, with hyperpyrexia, sometimes progressing to generalized rigidity and coma
10.2 Overdosage Management
There are no specific antidotes for PARNATE. For current information on the management of poisoning or overdosage, contact a poison control center at 1-800-222-1222.
Abrupt withdrawal of PARNATE following overdosage can precipitate withdrawal symptoms, including delirium [see Warnings and Precautions 5.9)and Drug Abuse and Dependence (9.3)].
Medical management should normally consist of general supportive measures, close observation of vital signs, and steps to counteract specific manifestations as they occur [see Warnings and Precautions (5)].The toxic effects of PARNATE may be delayed or prolonged following the last dose of the drug [see Clinical Pharmacology (12.2)]. Therefore, the patient should be closely observed for at least 1 week.
Data on the dialyzability of tranylcypromine are lacking.
11. Parnate Description
Tranylcypromine sulfate, the active ingredient of PARNATE, is a non-hydrazine MAOI. The chemical name is (±)‑trans‑2‑phenylcyclopropylamine sulfate (2:1). The molecular formula is (C9H11N)2•H2SO4 and its molecular weight is 364.46. The structural formula is:
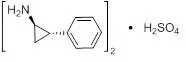
PARNATE film-coated tablets are intended for oral administration. Each round, rose‑red tablet is debossed on one side with the product name “PARNATE” and “SB” and contains tranylcypromine sulfate equivalent to 10 mg of tranylcypromine.
Inactive ingredients consist of microcrystalline cellulose, anhydrous citric acid, croscarmellose sodium, D&C Red No. 7, FD&C Blue No. 2, FD&C Yellow No. 6, gelatin, lactose, magnesium stearate, talc, titanium dioxide, carnauba wax, polyethylene glycol 400 and 8000, and hypromellose.
12. Parnate - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of PARNATE as an antidepressant is not fully understood, but is presumed to be linked to potentiation of monoamine neurotransmitter activity in the central nervous system (CNS) resulting from its irreversible inhibition of the enzyme monoamine oxidase (MAO).
16. How is Parnate supplied
PARNATE (tranylcypromine) tablets are available as:
- 10 mg: film-coated, round, rose-red and debossed with the product name “PARNATE” and “SB” on one side of the tablet containing tranylcypromine sulfate equivalent to 10 mg of tranylcypromine.
- Bottles of 100 tablets: NDC 59212-447-10
Store between 15° and 30°C (59° and 86°F). Dispense in a tight, light resistant container.
17. Patient Counseling Information
Advise the patient to read FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidal thoughts and behaviors, especially early during treatment and when the dosage is adjusted up or down [see Box Warning and Warnings and Precautions (5.1)].
Hypertensive Crisis
Advise the patient on possible symptoms and instruct the patient to seek immediate medical attention if related signs or symptoms are present [see Boxed Warning and Warnings and Precautions (5.2)]
Serotonin Syndrome
Advise the patient on possible symptoms, and explain the potentially fatal nature of serotonin syndrome and that it may result from an interaction with other serotonergic drugs. Instruct the patient to seek immediate medical attention if related signs or symptoms are present [see Warnings and Precautions (5.3)]
Interaction with Other Drugs and Dietary Supplements[see Contraindications (4.1)and Drug Interactions (7.1)]
- Warn the patient not to take concomitant medications, whether prescription or over‑the‑counter drugs, or dietary supplements without prior consultation with a health care provider able to provide advice on the potential for interactions.
- Explain to the patient that some other drugs may require a medication-free interval even after discontinuation of PARNATE.
- Advise the patient to inform other physicians, pharmacists, and dentists about the treatment with PARNATE.
Interaction with Foods and Beverages[see Contraindications (4.1)and Drug Interactions (7.2)]
- Warn the patient to avoid tyramine-rich foods and beverages.
- Advise the patient to avoid eating foods if storage conditions or freshness is unknown and to be cautious of foods of unknown age or composition even if refrigerated.
Hypotension
Advise the patient to report any symptoms of hypotension in the initial phase of treatment to the healthcare provider, because occurrence of such symptoms may require discontinuation [see Dosage and Administration (2.1) and Warnings and Precautions (5.5)].
Withdrawal Symptoms
Warn the patient not to stop PARNATE treatment abruptly, as withdrawal symptoms may occur and that the effect of PARNATE may continue even after discontinuation [see Warnings and Precautions (5.8, 5.9)].
Aggravation of Coexisting Symptoms of Depression
Inform the patient that PARNATE may aggravate coexisting symptoms in depression, such as anxiety and agitation and instruct them to contact their healthcare provider if they experience such symptoms [see Warnings and Precautions (5.13)].
Effects on Ability to Drive or Use Machinery[see Warnings and Precautions (5.14)]
- Warn the patient about the possible adverse reactions that can impair the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Tell the patient not to operate hazardous machinery and automobiles until they are reasonably certain that their ability to engage in such activities is not impaired.
Mfd. for:
Concordia Pharmaceuticals
Distributed by:
Amdipharm Limited
17 Northwood House
Dublin 9, Ireland
©2015 All rights reserved.
Medication Guide
| MEDICATION GUIDE
PARNATE® (PAR-nate) (tranylcypromine) Tablets |
What is the most important information I should know about PARNATE?
PARNATE can cause serious side effects including:
- Increase in suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment and when the PARNATE dose is changed. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.These include people who have, or have a family history of, bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions. PARNATE is not for use in children.
How can I watch for and try to prevent suicidal thoughts and actions?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms
Call a healthcare provider right away if you have any of the following symptoms, especially if they are new, worse, or worry you:
|
|
|
|
|
|
|
|
|
|
|
|
- A sudden, severe increase in blood pressure (hypertensive crisis). A hypertensive crisis can happen when you eat certain foods and drinks certain beverages during or after PARNATE treatment. A hypertensive crisis can lead to stroke and death. People who have thyroid problems (hyperthyroidism) may have a higher chance of having a hypertensive crisis. Symptoms of a hypertensive crisis may include:
|
|
|
|
|
|
|
|
|
|
A hypertensive crisis can also happen if you take PARNATE with certain other medicines. See, “Who should not take PARNATE?”
Avoid foods and drinks with a lot of tyramine while taking PARNATE and for 2 weeks after you stop taking it. For a list of some of the foods and drinks you should avoid during treatment with PARNATE see, “What should I avoid while taking PARNATE?”
What is PARNATE?
PARNATE is a prescription medicine used to treat adults with a certain type of depression called major depressive disorder (MDD) who have not responded well to treatment with other medicines used to treat depression (antidepressants). PARNATE belongs to a class of medicines called monoamine oxidase inhibitors (MAOIs).
- It is important to talk with your healthcare provider about the risks of treating depression and the risk of not treating it. Talk with your healthcare provider about all your treatment choices.
- PARNATE is not for use as the first medicine to treat MDD.
- It is not known if PARNATE is safe and effective for use in children.
Who should not take PARNATE?
Taking PARNATE with certain antidepressants and certain pain, allergy symptom, and cold and cough symptom medicines may cause a potentially life-threatening hypertensive crisis or a problem called serotonin syndrome. See, "What is the most important information I should know about PARNATE?” and “What are the possible side effects of PARNATE?”
Do not take PARNATE if you:
-
take certain medicines, including:
- antidepressants, such as:
- other monoamine oxidase inhibitors (MAOIs)
- selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs)
- tricyclic antidepressants
- other antidepressants, such as amoxapine, bupropion, maprotiline, nefazodone, trazodone, vilazodone, vortioxetine
- amphetamines and methylphenidates
- medicines that can raise blood pressure (sympathomimetic medicine), such as pseudoephedrine, phenylephrine and ephedrine. These medicines are in some cold, hay fever or weight-loss medicines.
- sympathomimetic herbal medicines or dietary supplements
- antihistamines (allergy medicines)
- triptans
- buspirone
- carbamazepine
- dextromethorphan
- dopamine
- hydroxytryptophan and tryptophan
- levodopa and methyldopa
- meperidine
- rasagline
- resperine
- s-adenosyl-L-methionine (SAM-e)
- tapentadol
- tetrabenazine
- antidepressants, such as:
Ask your healthcare provider or pharmacist if you are not sure if you take any of these medicines.
- have a tumor on your adrenal gland called a pheochromocytoma or a type of tumor called a paraganglioma.
Before taking PARNATE, tell your healthcare provider about all your medical conditions, including if you:
- have high or low blood pressure
- have heart problems
- have cerebrovascular problems or have had a stroke
- have headaches
- have, or have a family history of, bipolar disorder, mania, or hypomania
- plan to have surgery
- have liver or thyroid problems
- have or have had seizures or convulsions
- have diabetes
- are pregnant or plan to become pregnant. PARNATE may harm your unborn baby.
- are breastfeeding or plan to breastfeed. PARNATE passes into your breast milk. Do not breastfeed during treatment with PARNATE. Talk to your healthcare provider about the best way to feed your baby while taking PARNATE.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
PARNATE and some other medicines may affect each other causing serious side effects. PARNATE may affect the way other medicines work, and other medicines may affect how PARNATE works.
Some medicines need to be stopped for a period of time before you can start taking PARNATE and for a period of time after you stop taking PARNATE.
Know the medicines you take. Keep a list of them to show your healthcare providers, pharmacist, and dentist when you get a new medicine.
How should I take PARNATE?
- Take PARNATE exactly as your healthcare provider tells you to take it.
- Your healthcare provider may need to change your dose of PARNATE until it is the right dose for you.
- Do not stop taking PARNATE without first talking to your healthcare provider. Stopping PARNATE suddenly may cause withdrawal symptoms. See, “What are the possible side effects of PARNATE?”
- Tell your healthcare provider if you think your condition has gotten worse during treatment with PARNATE.
- If you take too much PARNATE (overdose) call your healthcare provider or poison control, or go to the nearest hospital emergency room right away.
What should I avoid while taking PARNATE?
- Do not eat foods or have drinks that have high amounts of tyramine while taking PARNATE or for 2 weeks after you stop taking PARNATE.
- All foods you eat should be fresh or properly frozen.
- Avoid foods when you do not know how those foods should be stored.
- Ask your healthcare provider if you are not sure if certain foods and drinks contain tyramine.
The table below lists some of the foods and drinks you should avoid while you take PARNATE.
| Type of Food and Drink that contain Tyramine
|
|
| Meat, Poultry, and Fish |
|
| Vegetables |
|
| Dairy (milk products) |
|
| Drinks |
|
| Other |
|
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how PARNATE affects you.
- Do not drink alcohol while taking PARNATE.
What are the possible side effects of PARNATE?
PARNATE may cause serious side effects, including:
- See “What is the most important information I should know about PARNATE?”
-
Serotonin Syndrome. A potentially life-threatening problem called serotonin syndrome can happen when you take PARNATE with certain other medicines. See, "Who should not take PARNATE?" Symptoms of serotonin syndrome may include:
- agitation, confusion
- seeing or hearing things that are not real (hallucinations)
- coma
- rapid pulse
- changes in blood pressure
- dizziness
- sweating
- flushing
- high body temperature (hyperthermia)
- fever
- seizures
- tremors, stiff muscles, or muscle twitching
- becoming unstable
- nausea, vomiting, diarrhea
If you have any of these symptoms, call your healthcare provider or go to the nearest hospital emergency room right away. -
Mania or hypomania (manic episodes) in people who have a history of bipolar disorder.
- greatly increased energy
- severe problems sleeping
- racing thoughts
- reckless behavior
- unusually grand ideas
- excessive happiness or irritability
- talking more or faster than usual
- Low blood pressure (hypotension) including a drop in your blood pressure when you stand or sit up (postural hypotension). This can happen more often in people who have high blood pressure (hypertension) and when the PARNATE dose is changed. Postural hypotension may cause you to feel dizzy and faint (syncope).
- Changes in your blood pressure (hypotension or hypertension) during surgery and during the time around surgery (perioperative). Taking PARNATE with certain medicines used for anesthesia can cause hypotension or hypertension. If you plan to have surgery, tell your surgeon or the healthcare provider who will give you anesthesia that you take PARNATE. Your healthcare provider should stop PARNATE at least 10 days before you have surgery.
Withdrawal symptoms. Talk with your healthcare provider before you stop taking PARNATE. Symptoms of withdrawal may include
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- Liver problems
- Seizures (convulsions). Seizures have happened in people who take too much PARNATE.
- Low blood sugar (hypoglycemia). Hypoglycemia has happened in people with diabetes who take medicines to lower blood sugar. Talk with your healthcare provider about checking your blood sugar during treatment with PARNATE. Tell your healthcare provider if your blood sugar gets low.
- Worsening of symptoms that can happen with depression, such as anxiety and agitation.
|
|
|
|
|
|
|
|
|
These are not all the side effects of PARNATE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store PARNATE?
- Store PARNATE between 59°F to 86°F (15°C to 30°C).
- Store PARNATE in a tight, light resistant container.
Keep PARNATE and all medicines out of the reach of children.
General information about the safe and effective use of PARNATE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take PARNATE for a condition for which it was not prescribed. Do not give PARNATE to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about PARNATE that is written for health professionals.
What are the ingredients in PARNATE?
Active Ingredient: tranylcypromine sulfate
Inactive Ingredients: microcrystalline cellulose, anhydrous citric acid, croscarmellose sodium, D&C Red No. 7, FD&C Blue No. 2, FD&C Yellow No. 6, gelatin, lactose, magnesium stearate, talc, titanium dioxide, carnauba wax, polyethylene glycol 400 and 8000 and hypromellose
Mfd. for:
Concordia Pharmaceuticals
Distributed by:
Amdipharm Limited
17 Northwood House
Dublin 9, Ireland
©2015 All rights reserved.
For more information, contact Concordia Pharmaceuticals at 1-877-370-1142.
| PARNATE
tranylcypromine sulfate tablet, film coated |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Concordia Pharmaceuticals Inc. (815240092) |





