Drug Detail:Pepaxto ( melphalan flufenamide)
Drug Class: Alkylating agents
Highlights of Prescribing Information
PEPAXTO® (melphalan flufenamide) for injection, for intravenous use
Initial U.S. Approval: 2021
Indications and Usage for Pepaxto
PEPAXTO is an alkylating drug indicated in combination with dexamethasone, for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy and whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent, and one CD38-directed monoclonal antibody. (1)
This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s). (1)
Limitations of Use: PEPAXTO is not indicated and is not recommended for use as a conditioning regimen for transplant outside of controlled clinical trials. (1, 5.5)
Pepaxto Dosage and Administration
- Recommended dosage of PEPAXTO is 40 mg intravenously over 30 minutes on Day 1 of each 28-day treatment cycle, in combination with dexamethasone. (2.1)
- See Full Prescribing Information for instructions on preparation and administration. (2.4)
Dosage Forms and Strengths
For injection: 20 mg melphalan flufenamide as a lyophilized powder in single-dose vial for reconstitution and dilution. (3)
Contraindications
History of serious hypersensitivity reaction to melphalan flufenamide or melphalan. (4)
Warnings and Precautions
- Thrombocytopenia: Monitor platelet counts at baseline, during treatment, and as clinically indicated. Dose delay or dose reduction may be required to allow recovery of platelets. (2.3, 5.1)
- Neutropenia: Monitor neutrophil counts at baseline, during treatment and as clinically indicated. Monitor patients with neutropenia for signs of infection. Dose delay or dose reduction may be required to allow recovery of neutrophils. (2.3, 5.2)
- Anemia: Monitor red blood cell counts at baseline, during treatment, and as clinically indicated. (5.3)
- Infections: Monitor for signs/symptoms of infection and treat promptly. (5.4)
- Increased Risk of Mortality with PEPAXTO at Dosages Higher than the Recommended Dosage: Dosages exceeding the recommended dose for PEPAXTO may be associated with mortality. (1, 5.5, 13.2)
- Secondary Malignancies: Monitor patients long-term for the development of secondary malignancies. (5.6)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.7, 8.1, 8.3)
Adverse Reactions/Side Effects
Most common adverse reactions (> 20%) are fatigue, nausea, diarrhea, pyrexia and respiratory tract infection. (6.1)
Most common laboratory abnormalities (≥50%) are leukocytes decrease, platelets decrease, lymphocytes decrease, neutrophils decrease, hemoglobin decrease and creatinine increase. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Oncopeptides Inc at 1-866-522-8894 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2021
Related/similar drugs
Darzalex, Blenrep, Tecvayli, Revlimid, Velcade, Pomalyst, KyprolisFull Prescribing Information
1. Indications and Usage for Pepaxto
PEPAXTO is indicated in combination with dexamethasone for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy and whose disease is refractory to at least one proteasome inhibitor, one immunomodulatory agent, and one CD38-directed monoclonal antibody.
This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s) [see Clinical Studies (14)].
2. Pepaxto Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of PEPAXTO is 40 mg administered intravenously over 30 minutes on Day 1 of each 28-day cycle until disease progression or until unacceptable toxicity. Administer dexamethasone 40 mg orally or intravenously on Days 1, 8, 15 and 22 of each cycle. For patients 75 years of age or older, reduce the dose of dexamethasone to 20 mg. Refer to the prescribing information for dexamethasone for additional dosing information [see Clinical Studies (14)].
2.2 Recommended Premedication and Concomitant Medications
Consider providing a serotonin-3 (5-HT3) receptor antagonist or other antiemetics prior to and during the treatment with PEPAXTO.
2.3 Dosage Modifications for Adverse Reactions
Withold PEPAXTO if the neutrophil count is less than 1 × 109/L or the platelet count is less than 50 × 109/L.
The recommended dose reductions and dosage modifications for adverse reactions for PEPAXTO are presented in Table 1 and Table 2, respectively.
| Dose Reduction | Dosage* |
|---|---|
|
|
| First | 30 mg |
| Second | 20 mg |
| Subsequent | Permanently discontinue PEPAXTO in patients who are unable to tolerate 20 mg. |
| Adverse Reaction | Severity | Dosage Modification |
|---|---|---|
| Myelosuppression [see Warnings and Precautions (5.1, 5.2)] | Platelet count less than 50 × 109/L on an intended PEPAXTO dosing day |
|
| Absolute neutrophil count less than 1 × 109/L on an intended PEPAXTO dosing day |
|
|
| Grade 4 hematological adverse reaction on an intended PEPAXTO dosing day in 2 consecutive cycles |
|
|
| Non-Hematologic Adverse Reaction [see Adverse Reactions (6.1)] | Grade 2 |
|
| Grade 3 or 4 |
|
2.4 Preparation and Administration
PEPAXTO is a hazardous drug. Follow applicable special handling and disposal procedures.1
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if visibly opaque particles, discoloration or foreign particles are observed.
Reconstitute and dilute PEPAXTO prior to infusion.
3. Dosage Forms and Strengths
For Injection: 20 mg melphalan flufenamide as a sterile lyophilized white to off-white powder in a single-dose vial for reconstitution and further dilution.
4. Contraindications
PEPAXTO is contraindicated in patients with a history of serious hypersensitivity reaction to melphalan flufenamide or melphalan [see Adverse Reactions (6.1)].
5. Warnings and Precautions
5.1 Thrombocytopenia
Thrombocytopenia was reported in 99% of 157 patients who received PEPAXTO with dexamethasone. Grade 3 thrombocytopenia was reported in 26% and Grade 4 thrombocytopenia was reported in 54% of patients [see Adverse Reactions (6.1)]. Thrombocytopenia may lead to hemorrhage. Any Grade hemorrhage was reported in 28% of 157 patients. Grade 3 hemorrhage was reported in 3.2% and Grade 4 hemorrhage was reported in <1% of patients [see Adverse Reactions (6.1)].
Grade 3 or 4 thrombocytopenia occurred in 43% of patients during the first cycle, with a median time to onset of 15 days from the first dose.
Monitor platelets at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment with PEPAXTO. Do not administer PEPAXTO if the platelet count is less than 50 × 109/L. Withhold PEPAXTO until platelet count 50 × 109/L or greater and resume at same or reduced dose based on duration of interruption. Adjust dose and/or dose schedule based on signs and symptoms of bleeding [see Dosage and Administration (2.3)].
5.2 Neutropenia
Neutropenia was reported in 95% of 157 patients who received PEPAXTO with dexamethasone. Grade 3 neutropenia was reported in 41% and Grade 4 neutropenia was reported in 40% of patients. Febrile neutropenia was reported in 6% of patients [see Adverse Reactions (6.1)]. Neutropenia may lead to infection.
Grade 3 or 4 neutropenia occurred in 50% during the first cycle, with a median time to onset of 15 days from the first dose.
Monitor neutrophil counts at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment with PEPAXTO. Do not administer PEPAXTO if absolute neutrophil count less than 1 × 109/L. Withhold PEPAXTO until absolute neutrophil count is 1 × 109/L or greater and resume at same or reduced dose based on duration of interruption [see Dosage and Administration (2.3)]. Consider leukocyte growth factor as clinically appropriate.
5.3 Anemia
Anemia was reported in 84% of 157 patients who received PEPAXTO with dexamethasone. Grade 3 anemia was reported in 50% of 157 patients [see Adverse Reactions (6.1)].
Monitor red blood cell counts at baseline, during treatment, and as clinically indicated. Monitor more frequently during the first two months of treatment with PEPAXTO. Treat anemia as clinically indicated and as per standard guidelines. Dosage modification and dose delay of PEPAXTO may be required to allow for recovery of red blood cells.
5.4 Infections
Fatal infections were reported in <1% of 157 patients who received PEPAXTO with dexamethasone. Any Grade infection was reported in 58% of 157 patients who received PEPAXTO and dexamethasone. Grade 3 infections were reported in 20% and Grade 4 infection was reported in 1.9% of patients. Respiratory tract infection occurred in 24% (Grade ≥3 in 5%), pneumonia in 13% (Grade ≥3 in 11%), and sepsis in 3.8% (Grade ≥3 in 3.2%) of patients [see Adverse Reactions (6.1)]. Consider antimicrobials as clinically appropriate.
5.5 Increased Risk of Mortality with PEPAXTO at Dosages Higher than the Recommended Dosage
A nonclinical safety study in dogs with melphalan flufenamide at dosages exceeding the recommended dose for relapsed or refractory multiple myeloma was associated with mortality [see Nonclinical Toxicology (13.2)]. There is limited clinical experience of PEPAXTO at dosages higher than recommended. The safety and efficacy of PEPAXTO has not been established for use as a conditioning regimen in patients receiving transplant.
5.6 Secondary Malignancies
Secondary malignancies such as myelodysplastic syndromes or acute leukemia have occurred in patients with multiple myeloma who have received PEPAXTO. Monitor patients long-term for the development of secondary malignancies.
5.7 Embryo-Fetal Toxicity
Based on its mechanism of action, PEPAXTO can cause fetal harm when administered to a pregnant woman because it is genotoxic and targets actively dividing cells. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with PEPAXTO and for 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with PEPAXTO and for 3 months after the last dose [see Use In Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Thrombocytopenia [see Warnings and Precautions (5.1)].
- Neutropenia [see Warnings and Precautions (5.2)].
- Anemia [see Warnings and Precautions (5.3)].
- Infections [see Warnings and Precautions (5.4)].
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
PEPAXTO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of PEPAXTO have not been established in pediatric patients.
8.5 Geriatric Use
Of the 157 patients with RRMM who received PEPAXTO, 50% were 65 years and older, while 16% were 75 years and older. No overall differences in safety were observed between these patients and younger patients. Clinical studies of PEPAXTO in patients with RRMM did not include sufficient numbers of patients 65 years of age and older to determine if they respond differently from younger adult patients.
11. Pepaxto Description
Melphalan flufenamide is an alkylating drug. The chemical name is Ethyl (2S)-2-[[(2S)-2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoyl]amino]-3-(4-fluorophenyl)propanoate hydrochloride and the molecular weight is 498.4 as free base and 534.9 as the hydrochloride salt. The structural formula is:
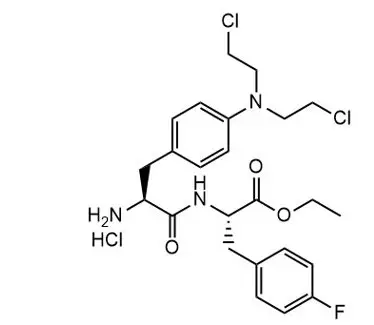
Melphalan flufenamide hydrochloride is soluble in most organic solvents, while sparsely soluble in aqueous solutions. The pKa value is 7.13.
PEPAXTO for injection is supplied as a sterile, white to off-white lyophilized powder in a single-dose vial for intravenous use. Each vial contains 20 mg melphalan flufenamide (equivalent to 21.48 mg melphalan flufenamide hydrochloride) and 1,000 mg sucrose.
12. Pepaxto - Clinical Pharmacology
12.1 Mechanism of Action
Melphalan flufenamide is a peptide conjugated alkylating drug. Due to its lipophilicity, melphalan flufenamide is passively distributed into cells and thereafter enzymatically hydrolyzed to melphalan. Similar to other nitrogen mustard drugs, cross-linking of DNA is involved in the antitumor activity of melphalan flufenamide. In cellular assays, melphalan flufenamide inhibited proliferation and induced apoptosis of hematopoietic and solid tumor cells. Additionally, melphalan flufenamide showed synergistic cytotoxicity with dexamethasone in melphalan resistant and non-resistant multiple myeloma cell lines.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of PEPAXTO have not been fully characterized.
12.3 Pharmacokinetics
Melphalan flufenamide peak plasma concentrations were reached during the 30-minute infusion. Peak plasma concentrations of the active metabolite melphalan were reached 4 to 15 minutes after the end of infusion of PEPAXTO 40 mg. Following PEPAXTO 40 mg, the mean (CV%) Cmax was 432 ng/mL (30%) and AUC0-INF was 3,143 µg/mL∙hr (28%) for melphalan after a single dose. The mean (CV%) Cmax was 419 ng/mL (33%) and AUC0-INF was 2,933 µg/mL∙hr (29%) for melphalan at steady-state.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies have been conducted with melphalan flufenamide.
PEPAXTO is genotoxic. In studies conducted in vitro, melphalan flufenamide caused irreversible DNA damage.
Repeat-dose toxicity studies with melphalan flufenamide in animals showed adverse effects on male reproductive organs. Melphalan flufenamide was administered intravenously to rats at 20, 40, or 55 mg/m2, and to dogs at 0.45 or 0.90 mg/kg (9 or 18 mg/m2) every 21 days for two or three doses. Decreased testes weights and depletion of germ cells were observed in both species, and epididymal oligospermia was observed in dogs. Adverse effects on male reproductive organs were observed in dogs at dose levels less than the recommended clinical dose of 40 mg. The reversibility of adverse effects on male reproductive organs was not assessed.
13.2 Animal Toxicology and/or Pharmacology
Dogs were intravenously administered a single dose of melphalan flufenamide (17.5 mg/kg) or an equimolar dose of melphalan; these dose levels were representative of dosages needed for myeloablation. Increased mortality was observed in dogs administered melphalan flufenamide despite similar melphalan exposure in animals administered melphalan flufenamide or melphalan.
14. Clinical Studies
The efficacy of PEPAXTO in combination with dexamethasone was evaluated in HORIZON [NCT02963493], a multicenter, single-arm trial. Eligible patients were required to have relapsed or refractory multiple myeloma. Patients received PEPAXTO 40 mg intravenously on Day 1 and dexamethasone 40 mg orally (20 mg for patients ≥75 years of age) on Day 1, 8, 15 and 22 of each 28-day cycle until disease progression or unacceptable toxicity.
A total of 157 patients accepting a central venous catheter and with estimated creatinine clearance by Cockcroft-Gaut formula ≥45 mL/min were enrolled. Patients with primary refractory disease (i.e. never responded with at least minimal response to any prior treatment) were excluded. Ninety seven patients had received four or more prior lines of therapies and were refractory to at least one proteasome inhibitor, at least one immunomodulatory agent and a CD38-directed monoclonal antibody. The median age was 65 years (range: 35 to 86 years); 58% were male, 87% were White and 6% were Black or African American. Disease characteristics in these 97 patients are summarized in Table 5.
The major efficacy outcome measure was overall response rate (ORR) and Duration of Response (DoR) assessed according to the International Myeloma Working Group (IMWG) Criteria by investigators. Efficacy results in the 97 patients are provided in Table 6. The median time to first response was 2.1 months (range: 1.0 to 6.1 months).
| Parameter | PEPAXTO with Dexamethasone (N=97) |
|---|---|
|
|
| Years from diagnosis to start of PEPAXTO, median (range) | 6.4 (2.1 to 24.6) |
| Prior treatment regimens, median (range) | 6 (4 to 12) |
| Documented refractory status, (%) | |
| Lenalidomide | 94 |
| Pomalidomide | 92 |
| Bortezomib | 74 |
| Carfilzomib | 63 |
| Daratumumab | 93 |
| Alkylator refractory, (%) | 75 |
| Previous stem cell transplant, (%) | 70 |
| International Staging System at Baseline, (%) | |
| I | 30 |
| II | 32 |
| III | 34 |
| Missing/Unknown | 4 |
| High-risk cytogenetics*, (%) | 33 |
| Extramedullary disease (EMD), (%) | 41 |
| PEPAXTO with Dexamethasone (N=97) |
|
|---|---|
| Overall response rate (ORR), n (%) (95% CI) | 23 (23.7) (15.7, 33.4) |
| Stringent complete response (sCR) | 0 |
| Complete Response (CR) | 0 |
| Very good partial response (VGPR), n (%) | 9 (9.3) |
| Partial response (PR), n (%) | 14 (14.4) |
| Median duration of response in months (95% CI) | 4.2 (3.2, 7.6) |
| PEPAXTO
melphalan flufenamide injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Oncopeptides, AB (632402728) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Magle Chemoswed AB | 559533158 | API MANUFACTURE(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cenexi-Laboratoires Thissen SA (Cenexi LT) | 370088959 | MANUFACTURE(73657-020) , ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotek Produktion & Laboratorier AB (APL) | 632474078 | ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Biopharma Product Testing, Denmark A/S (Eurofins) | 311900950 | ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Solvias AG (Solvias) | 480739627 | ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmascience Inc. | 202657094 | ANALYSIS(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc.; "PCI of Illinois" | 098908572 | LABEL(73657-020) , PACK(73657-020) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| West Pharmaceutical Services, Inc. | 002330983 | ANALYSIS(73657-020) | |





