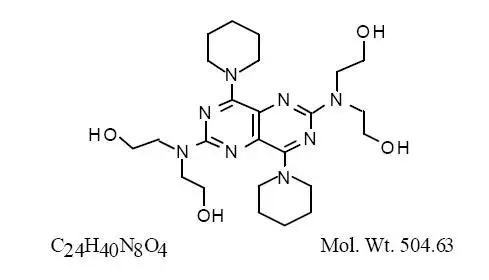
Drug Detail:Persantine (Dipyridamole (oral/injection) [ dye-pir-id-a-mole ])
Drug Class: Cardiac stressing agents Platelet aggregation inhibitors
Related/similar drugs
aspirin, warfarin, Coumadin, Lexiscan, Adenocard, Ecotrin, JantovenIndications and Usage for Persantine
Precautions
Adverse Reactions/Side Effects
| Adverse Reaction | PERSANTINE Tablets / Warfarin | Placebo / Warfarin |
| Number of patients | 147 | 170 |
| Dizziness | 13.6% | 8.2% |
| Abdominal distress | 6.1% | 3.5% |
| Headache | 2.3% | 0.0% |
| Rash | 2.3% | 1.1% |
| PERSANTINE
dipyridamole tablet, coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| PERSANTINE
dipyridamole tablet, coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| PERSANTINE
dipyridamole tablet, coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Promeco, S.A. de C.V. | 812579472 | ANALYSIS(0597-0017, 0597-0019, 0597-0018) , MANUFACTURE(0597-0017, 0597-0019, 0597-0018) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Malgrat Pharma Chemicals, S.L.U. | 468215759 | API MANUFACTURE(0597-0017, 0597-0018, 0597-0019) | |