Drug Class: Bacterial vaccines
Highlights of Prescribing Information
PNEUMOVAX® 23 (pneumococcal vaccine polyvalent)
Sterile, Liquid Vaccine for Intramuscular or Subcutaneous
Injection
Initial U.S. Approval: 1983
Indications and Usage for Pneumovax 23
PNEUMOVAX 23 is a vaccine indicated for active immunization for the prevention of pneumococcal disease caused by the 23 serotypes contained in the vaccine (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F). (1.1)
PNEUMOVAX 23 is approved for use in persons 50 years of age or older and persons aged ≥2 years who are at increased risk for pneumococcal disease. (1.1, 14.1)
Pneumovax 23 Dosage and Administration
Single 0.5-mL dose of PNEUMOVAX 23 administered intramuscularly or subcutaneously only. (2.2)
Dosage Forms and Strengths
Clear, sterile solution supplied in a (0.5-mL dose) single-dose vial and a single-dose, prefilled syringe. (3)
Contraindications
Severe allergic reaction (e.g., anaphylaxis) to any component of PNEUMOVAX 23. (4.1)
Warnings and Precautions
- Use caution and appropriate care for individuals with severely compromised cardiovascular and/or pulmonary function in whom a systemic reaction would pose a significant risk. (5.2)
Adverse Reactions/Side Effects
The most common adverse reactions, reported in >10% of subjects vaccinated with PNEUMOVAX 23 for the first time in a clinical trial, were: injection-site pain/soreness/tenderness (60.0%), injection-site swelling/induration (20.3%), headache (17.6%), injection-site erythema (16.4%), asthenia and fatigue (13.2%), and myalgia (11.9%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
Drug Interactions
In a randomized clinical study, a reduced immune response to ZOSTAVAX® as measured by gpELISA was observed in individuals who received concurrent administration of PNEUMOVAX 23 and ZOSTAVAX compared with individuals who received these vaccines 4 weeks apart. Consider administration of the two vaccines separated by at least 4 weeks. (7.1, 14.3)
Use In Specific Populations
Pediatrics: PNEUMOVAX 23 is not approved for use in children younger than 2 years of age because children in this age group do not develop an effective immune response to capsular types contained in the polysaccharide vaccine. (8.4)
Geriatrics: For subjects aged 65 years or older in a clinical study systemic adverse reactions, determined by the investigator to be vaccine-related, were higher following revaccination (33.1%) than following initial vaccination (21.7%). Routine revaccination of immunocompetent persons previously vaccinated with a 23-valent vaccine, is not recommended. (8.5)
Immunocompromised Individuals: Response to vaccination may be diminished. (5.4, 8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2023
Related/similar drugs
Prevnar, Prevnar 13, Prevnar 20, pneumococcal 13-valent vaccine, VaxneuvanceFull Prescribing Information
1. Indications and Usage for Pneumovax 23
1.1 Indications and Use
PNEUMOVAX® 23 is a vaccine indicated for active immunization for the prevention of pneumococcal disease caused by the 23 serotypes contained in the vaccine (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F). PNEUMOVAX 23 is approved for use in persons 50 years of age or older and persons aged ≥2 years who are at increased risk for pneumococcal disease.
2. Pneumovax 23 Dosage and Administration
For intramuscular or subcutaneous injection only.
2.1 Preparation
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. If either of these two conditions exists, the vaccine should not be administered.
- Do not mix PNEUMOVAX 23 with other vaccines in the same syringe or vial.
- Use a separate sterile syringe and needle for each individual patient to prevent transmission of infectious agents from one person to another.
Single-Dose Vial
Withdraw 0.5 mL from the vial using a sterile needle and syringe free of preservatives, antiseptics, and detergents.
Single-Dose, Prefilled Syringe
The package does not contain a needle. Attach a sterile needle to the prefilled syringe by twisting in a clockwise direction until the needle fits securely on the syringe.
2.2 Administration
Administer PNEUMOVAX 23 intramuscularly or subcutaneously into the deltoid muscle or lateral mid-thigh. Do not inject intravascularly or intradermally.
2.3 Revaccination
The Advisory Committee on Immunization Practices (ACIP) has recommendations for revaccination against pneumococcal disease for persons at high risk who were previously vaccinated with PNEUMOVAX 23. Routine revaccination of immunocompetent persons previously vaccinated with a 23-valent vaccine, is not recommended.
3. Dosage Forms and Strengths
PNEUMOVAX 23 is a clear, sterile solution supplied in a (0.5-mL dose) single-dose vial and a single-dose, prefilled syringe. [See Description (11) and How Supplied/Storage and Handling (16).]
5. Warnings and Precautions
5.1 Persons with Moderate or Severe Acute Illness
Defer vaccination with PNEUMOVAX 23 in persons with moderate or severe acute illness.
5.2 Persons with Severely Compromised Cardiovascular or Pulmonary Function
Caution and appropriate care should be exercised in administering PNEUMOVAX 23 to individuals with severely compromised cardiovascular and/or pulmonary function in whom a systemic reaction would pose a significant risk.
5.3 Use of Antibiotic Prophylaxis
This vaccine does not replace the need for penicillin (or other antibiotic) prophylaxis against pneumococcal infection. In patients who require penicillin (or other antibiotic) prophylaxis against pneumococcal infection, such prophylaxis should not be discontinued after vaccination with PNEUMOVAX 23.
6. Adverse Reactions/Side Effects
The most common adverse reactions, reported in >10% of subjects vaccinated with PNEUMOVAX 23 for the first time in a clinical trial, were: injection-site pain/soreness/tenderness (60.0%), injection-site swelling/induration (20.3%), headache (17.6%), injection-site erythema (16.4%), asthenia/fatigue (13.2%), and myalgia (11.9%). [See Adverse Reactions (6.1).]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
Primary Vaccination and Revaccination with PNEUMOVAX 23 in Adults 50 Years of Age or Older
In a randomized, double-blind, placebo-controlled crossover clinical trial, subjects were enrolled in four different cohorts defined by age (50-64 years of age and ≥65 years of age) and vaccination status (no pneumococcal vaccination or receipt of a pneumococcal polysaccharide vaccine 3-5 years prior to the study). Subjects in each cohort were randomized to receive intramuscular injections of PNEUMOVAX 23 followed by placebo (saline containing 0.25% phenol), or placebo followed by PNEUMOVAX 23, at 30-day (±7 days) intervals. The safety of an initial vaccination (first dose) was compared to revaccination (second dose) with PNEUMOVAX 23 for 14 days following each vaccination.
All 1008 subjects (average age, 67 years; 49% male and 51% female; 91% Caucasian, 4.7% African-American, 3.5% Hispanic, and 0.8% Other) received placebo injections.
Initial vaccination was evaluated in a total of 444 subjects (average age 65 years; 32% male and 68% female; 93% Caucasian, 3.2% African-American, 3.4% Hispanic, and 1.1% Other).
Revaccination was evaluated in 564 subjects (average age 69 years; 53% male and 47% female; 90% Caucasian, 3.5% Hispanic, 6.0% African-American, and 0.5% Other).
Solicited and Unsolicited Reactions
Table 1 presents the adverse event rates for all solicited and unsolicited reactions reported in ≥1% in any group in this study, without regard to causality.
The most common local adverse reactions reported at the injection site after initial vaccination with PNEUMOVAX 23 were pain/tenderness/soreness (60.0%), swelling/induration (20.3%), and erythema (16.4%). The most common systemic adverse experiences were headache (17.6%), asthenia/fatigue (13.2%), and myalgia (11.9%).
The most common local adverse reactions reported at the injection site after revaccination with PNEUMOVAX 23 were pain/soreness/tenderness (77.2%), swelling (39.8%), and erythema (34.5%). The most common systemic adverse reactions with revaccination were headache (18.1%), asthenia/fatigue (17.9%), and myalgia (17.3%). All of these adverse reactions were reported at a rate lower than 10% after receiving a placebo injection.
| PNEUMOVAX 23 Initial Vaccination | PNEUMOVAX 23 Revaccination* | Placebo Injection† | |
|---|---|---|---|
| N=444 | N=564 | N=1008 | |
|
|||
| Number Followed for Safety | 438 | 548 | 984‡ |
| AE Rate | AE Rate | AE Rate | |
| Injection-Site Complaints | |||
| Solicited Events | |||
| Pain/Soreness/Tenderness | 60.0% | 77.2% | 7.7% |
| Swelling/Induration | 20.3% | 39.8% | 2.8% |
| Erythema | 16.4% | 34.5% | 3.3% |
| Unsolicited Events | |||
| Ecchymosis | 0% | 1.1% | 0.3% |
| Pruritus | 0.2% | 1.6% | 0.0% |
| Systemic Complaints | |||
| Solicited Events | |||
| Asthenia/Fatigue | 13.2% | 17.9% | 6.7% |
| Chills | 2.7% | 7.8% | 1.8% |
| Myalgia | 11.9% | 17.3% | 3.3% |
| Headache | 17.6% | 18.1% | 8.9% |
| Unsolicited Events | |||
| Fever§ | 1.4% | 2.0% | 0.7% |
| Diarrhea | 1.1% | 0.7% | 0.5% |
| Dyspepsia | 1.1% | 1.1% | 0.9% |
| Nausea | 1.8% | 1.8% | 0.9% |
| Back Pain | 0.9% | 0.9% | 1.0% |
| Neck Pain | 0.7% | 1.5% | 0.2% |
| Upper Respiratory Infection | 1.8% | 2.6% | 1.8% |
| Pharyngitis | 1.1% | 0.4% | 1.3% |
In this clinical study an increased rate of local reactions was observed with revaccination at 3-5 years following initial vaccination.
For subjects aged 65 years or older, injection-site adverse reaction rate was higher following revaccination (79.3%) than following initial vaccination (52.9%). The proportion of subjects reporting injection site discomfort that interfered with or prevented usual activity or injection site induration ≥4 inches was higher following revaccination (30.6%) than following initial vaccination (10.4%). Injection site reactions typically resolved by 5 days following vaccination.
For subjects aged 50-64 years, the injection-site adverse reaction rate for revaccinees and initial vaccinees was similar (79.6% and 72.8% respectively).
The rate of systemic adverse reactions was similar among both initial vaccinees and revaccinees within each age group. The rate of vaccine-related systemic adverse reactions was higher following revaccination (33.1%) than following initial vaccination (21.7%) in subjects 65 years of age or older, and was similar following revaccination (37.5%) and initial vaccination (35.5%) in subjects 50-64 years of age. The most common systemic adverse reactions reported after PNEUMOVAX 23 were as follows: asthenia/fatigue, myalgia and headache.
Regardless of age, the observed increase in post vaccination use of analgesics (≤13% in the revaccinees and ≤4% in the initial vaccinees) returned to baseline by day 5.
Sequential Administration of Prevnar 13 and PNEUMOVAX 23
In a randomized, double-blind, placebo-controlled, multicenter study, healthy adults, 50 years of age and older, received Prevnar 13 followed by PNEUMOVAX 23 either 8 weeks later (Group 1) or 26 weeks later (Group 2). Placebo was administered instead of PNEUMOVAX 23 at 26 weeks (Group 1) or 8 weeks (Group 2). Solicited injection site adverse reactions were evaluated during Days 1 through 5 postvaccination. Solicited systemic adverse reactions and any other adverse reactions were evaluated during Days 1 through 14 postvaccination, and any serious adverse events (SAEs) were collected throughout the study period (through Week 30). [See Clinical Studies (14.2).]
Overall, subjects were a mean age of 64.2 years (range: 50 to 97 years). There were more females (n=219, 54.8%) than males (n=181, 45.3%). By race, 84.8% of subjects were White, 9.3% were Black or African-American, and 6.1% were other racial groups; the majority of subjects were not Hispanic or Latino (n=322, 80.5%).
Serious Adverse Reactions
There were 24 SAEs reported in 20 subjects (n=9 [4.5%] Group 1; n=11 [5.5%] Group 2). No SAEs were considered related to vaccination.
Solicited Adverse Reactions
Solicited injection site adverse reactions that occurred during Days 1 through 5 postvaccination with PNEUMOVAX 23, solicited systemic adverse reactions that occurred during Days 1 through 14, and fever that occurred during Days 1 through 5 postvaccination with PNEUMOVAX 23 are presented in Table 2. In this study, 81.4% of subjects in Group 1 and 64.0% of subjects in Group 2 reported at least 1 injection site adverse reaction from Days 1 through 5 postvaccination with PNEUMOVAX 23, and 64.9% of subjects in Group 1 and 54.9% of subjects in Group 2 reported at least 1 systemic adverse reaction from Days 1 through 14 postvaccination with PNEUMOVAX 23.
| Group 1*
(Prevnar 13 -> PNEUMOVAX 23 -> Placebo) | Group 2†
(Prevnar 13 -> Placebo -> PNEUMOVAX 23) |
|||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Every subject is counted a single time for each applicable row and column. | ||||
| A specific adverse reaction appears in this table only if its incidence in one or more of the columns meets the incidence criterion in the table title, after rounding. | ||||
|
||||
| Injection Site Adverse Reactions | ||||
| Subjects in population with follow-up | 188 | 164 | ||
| Any injection site reaction | 153 | (81.4) | 105 | (64.0) |
| Any Injection site pain‡ | 149 | (79.3) | 105 | (64.0) |
| Mild | 72 | (38.3) | 65 | (39.6) |
| Moderate | 65 | (34.6) | 36 | (22.0) |
| Severe§ | 12 | (6.4) | 4 | (2.4) |
| Any Injection site swelling | 95 | (50.5) | 48 | (29.3) |
| 0 to <2.5 cm | 28 | (14.9) | 19 | (11.6) |
| ≥2.5 to <5.1 cm | 20 | (10.6) | 9 | (5.5) |
| ≥5.1 to <7.6 cm | 20 | (10.6) | 10 | (6.1) |
| ≥7.6 to <10.2 cm | 15 | (8.0) | 2 | (1.2) |
| ≥10.2 cm§ | 12 | (6.4) | 8 | (4.9) |
| Any Injection site erythema | 78 | (41.5) | 48 | (29.3) |
| 0 to <2.5 cm | 26 | (13.8) | 20 | (12.2) |
| ≥2.5 to <5.1 cm | 12 | (6.4) | 13 | (7.9) |
| ≥5.1 to <7.6 cm | 12 | (6.4) | 6 | (3.7) |
| ≥7.6 to <10.2 cm | 7 | (3.7) | 3 | (1.8) |
| ≥10.2 cm | 19 | (10.1) | 6 | (3.7) |
| Unknown [missing data] | 2 | (1.1) | 0 | (0.0) |
| Systemic Adverse Reactions | ||||
| Subjects in population with follow-up | 188 | 164 | ||
| Any systemic adverse reaction | 122 | (64.9) | 90 | (54.9) |
| Myalgia | 93 | (49.5) | 70 | (42.7) |
| Fatigue | 59 | (31.4) | 45 | (27.4) |
| Headache | 46 | (24.5) | 30 | (18.3) |
| Arthralgia | 37 | (19.7) | 25 | (15.2) |
| Subjects with temperature data¶ | 185 | 161 | ||
| Temperature ≥ 100.4°F | 1 | (0.5) | 0 | (0.0) |
6.2 Post-Marketing Experience
The following list of adverse reactions includes those identified during post approval use of PNEUMOVAX 23. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or their causal relationship to product exposure.
General disorders and administration site conditions
Cellulitis
Malaise
Fever (>102°F)
Warmth at the injection site
Decreased limb mobility
Peripheral edema in the injected extremity
Injection-site necrosis
Digestive System
Nausea
Vomiting
Hematologic/Lymphatic
Lymphadenitis
Lymphadenopathy
Thrombocytopenia in patients with stabilized idiopathic thrombocytopenic purpura
Hemolytic anemia in patients who have had other hematologic disorders
Leukocytosis
Hypersensitivity reactions including
Anaphylactoid reactions
Serum Sickness
Angioneurotic edema
Musculoskeletal System
Arthralgia
Arthritis
Nervous System
Paresthesia
Radiculoneuropathy
Guillain-Barré syndrome
Febrile convulsion
Skin
Rash
Urticaria
Cellulitis-like reactions
Erythema multiforme
Investigations
Increased serum C-reactive protein
7. Drug Interactions
7.1 Concomitant Administration with Other Vaccines
In a randomized clinical study, a reduced immune response to ZOSTAVAX® as measured by gpELISA was observed in individuals who received concurrent administration of PNEUMOVAX 23 and ZOSTAVAX compared with individuals who received these vaccines 4 weeks apart. Consider administration of the two vaccines separated by at least 4 weeks. [See Clinical Studies (14.3).]
Limited safety and immunogenicity data from clinical trials are available on the concurrent administration of PNEUMOVAX 23 and vaccines other than ZOSTAVAX.
8. Use In Specific Populations
8.4 Pediatric Use
PNEUMOVAX 23 is not approved for use in children less than 2 years of age. Children in this age group do not develop an effective immune response to the capsular types contained in this polysaccharide vaccine.
The ACIP has recommendations for use of PNEUMOVAX 23 in children 2 years of age or older, who have previously received pneumococcal vaccines, and who are at increased risk for pneumococcal disease.
8.5 Geriatric Use
In one clinical trial of PNEUMOVAX 23, conducted post-licensure, a total of 629 subjects who were aged ≥65 years and 201 subjects who were aged ≥75 years were enrolled.
In this trial, the safety of PNEUMOVAX 23 in adults 65 years of age and older (N=629) was compared to the safety of PNEUMOVAX 23 in adults 50 to 64 years of age (N=379). The subjects in this study had underlying chronic illness but were in stable condition; at least 1 medical condition at enrollment was reported by 86.3% of subjects who were 50 to 64 years old, and by 96.7% of subjects who were 65 to 91 years old. The rate of vaccine-related systemic adverse experiences was higher following revaccination (33.1%) than following primary vaccination (21.7%) in subjects ≥65 years of age, and was similar following revaccination (37.5%) and primary vaccination (35.5%) in subjects 50 to 64 years of age.
Since elderly individuals may not tolerate medical interventions as well as younger individuals, a higher frequency and/or a greater severity of reactions in some older individuals cannot be ruled out.
Post-marketing reports have been received in which some elderly individuals had severe adverse experiences and a complicated clinical course following vaccination. Some individuals with underlying medical conditions of varying severity experienced local reactions and fever associated with clinical deterioration requiring hospital care.
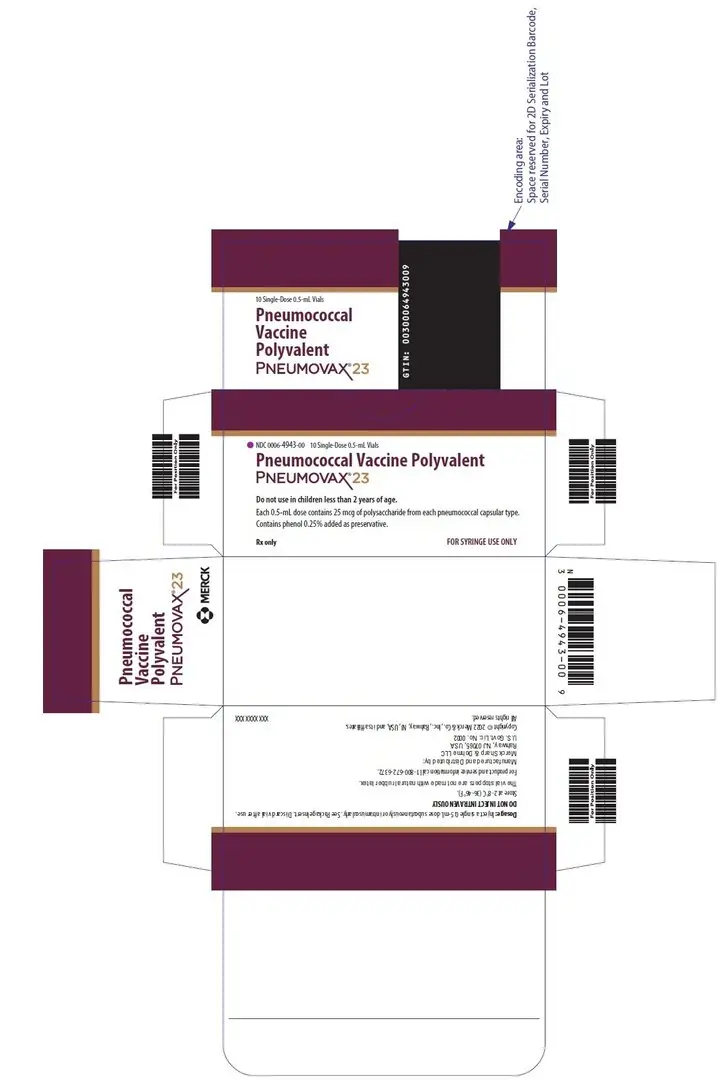
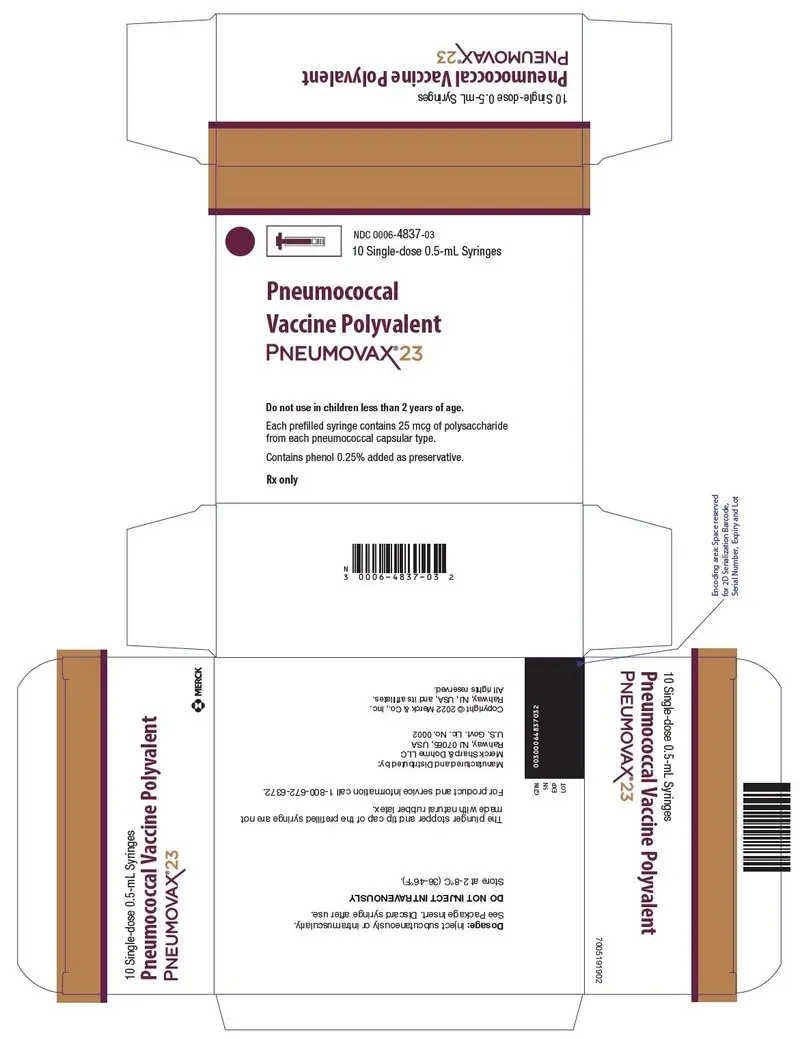
11. Pneumovax 23 Description
PNEUMOVAX 23 (Pneumococcal Vaccine Polyvalent) is a sterile, liquid vaccine consisting of a mixture of purified capsular polysaccharides from Streptococcus pneumoniae types (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F).
PNEUMOVAX 23 is a clear, colorless solution. Each 0.5-mL dose of vaccine contains 25 micrograms of each polysaccharide type in isotonic saline solution containing 0.25% phenol as a preservative. The vaccine is used directly as supplied. No dilution or reconstitution is necessary.
The vial stoppers, syringe plunger stopper and syringe tip cap are not made with natural rubber latex.
14. Clinical Studies
14.1 Effectiveness
The protective efficacy of pneumococcal vaccines containing six (types 1, 2, 4, 8, 12F, and 25) or twelve (types 1, 2, 3, 4, 6A, 8, 9N, 12F, 25, 7F, 18C, and 46) capsular polysaccharides was investigated in two controlled studies in South Africa in male novice gold miners ranging in age from 16 to 58 years, in whom there was a high attack rate for pneumococcal pneumonia and bacteremia. In both studies, participants in the control groups received either meningococcal polysaccharide serogroup A vaccine or saline placebo. In both studies, attack rates for vaccine type pneumococcal pneumonia were observed for the period from 2 weeks through about 1 year after vaccination. Protective efficacy was 76% and 92%, respectively, for the 6- and 12-valent vaccines, for the capsular types represented.
Three similar studies in South African young adult male novice gold miners were carried out by Dr. R. Austrian and associates using similar pneumococcal vaccines prepared for the National Institute of Allergy and Infectious Diseases, with pneumococcal vaccines containing a 6-valent formulation (types 1, 3, 4, 7, 8, and 12) or a 13-valent formulation (types 1, 2, 3, 4, 6, 7, 8, 9, 12, 14, 18, 19, and 25) capsular polysaccharides. The reduction in pneumococcal pneumonia caused by the capsular types contained in the vaccines was 79%. Reduction in type-specific pneumococcal bacteremia was 82%.
A prospective study in France found a pneumococcal vaccine containing fourteen (types 1, 2, 3, 4, 6A, 7F, 8, 9N, 12F, 14, 18C, 19F, 23F, and 25) capsular polysaccharides to be 77% (95%CI: 51% to 89%) effective in reducing the incidence of pneumonia among male and female nursing home residents with a mean age of 74 (standard deviation of 4 years).
In a study using a pneumococcal vaccine containing eight (types 1, 3, 6, 7, 14, 18, 19, and 23) capsular polysaccharides, vaccinated children and young adults aged 2 to 25 years who had sickle cell disease, congenital asplenia, or undergone a splenectomy experienced significantly less bacteremic pneumococcal disease than patients who were not vaccinated.
In the United States, one post-licensure randomized controlled trial, in the elderly or patients with chronic medical conditions who received a 14-valent pneumococcal polysaccharide vaccine (types 1, 2, 3, 4, 6A, 8, 9N, 12F, 14, 19F, 23F, 25, 7F, and 18C), did not support the efficacy of the vaccine for nonbacteremic pneumonia.
A retrospective cohort analysis study based on the U.S. Centers for Disease Control and Prevention (CDC) pneumococcal surveillance system, showed 57% (95%CI: 45% to 66%) overall protective effectiveness against invasive infections caused by serotypes included in PNEUMOVAX 23 in persons ≥6 years of age, 65 to 84% effectiveness among specific patient groups (e.g., persons with diabetes mellitus, coronary vascular disease, congestive heart failure, chronic pulmonary disease, and anatomic asplenia) and 75% (95%CI: 57% to 85%) effectiveness in immunocompetent persons aged ≥65 years of age. Vaccine effectiveness could not be confirmed for certain groups of immunocompromised patients.
14.2 Immunogenicity
The levels of antibodies that correlate with protection against pneumococcal disease have not been clearly defined.
Antibody responses to most pneumococcal capsular types are generally low or inconsistent in children less than 2 years of age.
Sequential Administration of Prevnar 13 and PNEUMOVAX 23
In a randomized, double-blind, placebo-controlled, multicenter study, healthy adults, 50 years of age and older, received Prevnar 13 followed by PNEUMOVAX 23 either 8 weeks later (Group 1) or 26 weeks later (Group 2). Four hundred subjects were randomized 1:1 into Group 1 or Group 2, all of whom were initially vaccinated with Prevnar 13; of these, 188 subjects received PNEUMOVAX 23 (Group 1) and 185 subjects received placebo (Group 2) at Week 8, and 172 subjects received placebo (Group 1) and 164 subjects received PNEUMOVAX 23 (Group 2) at Week 26.
Opsonophagocytic activity (OPA) titers were measured at prevaccination, at Week 12 and at Week 30 for the 12 shared serotypes contained in both PNEUMOVAX 23 and Prevnar 13 (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F), 2 of the 11 serotypes unique to PNEUMOVAX 23 (22F and 33F), and 1 serotype unique to Prevnar 13 (6A). OPA testing was performed on evaluable serum samples from all subjects at baseline (Day 1) and Week 12, and on sera from a random subset of subjects (approximately 50% of subjects) at Week 30. Estimated GMTs, GMT ratio, and 95% confidence intervals were obtained from a constrained Longitudinal Data Analysis model {1}.
For each of the shared serotypes, Week 12 OPA geometric mean titers (GMTs) in Group 1 were noninferior to those of Group 2, as the lower bounds of the 95% CIs for the OPA GMT ratios were >0.5 for all 12 shared serotypes. For serotypes 22F and 33F, OPA GMTs in Group 1 at Week 12 were superior to those of Group 2 at Week 12, as the lower bounds of the 95% CIs for the OPA GMT ratios were >2.0 for both serotypes.
The OPA GMTs to the 12 shared serotypes and 2 unique serotypes (22F and 33F) when measured 4 weeks after dosing with PNEUMOVAX 23 were generally similar between Group 1 (Week 12) and Group 2 (Week 30 subset).
14.3 Concomitant Administration with Other Vaccines
In a double-blind, controlled clinical trial, 473 adults, 60 years of age or older, were randomized to receive ZOSTAVAX and PNEUMOVAX 23 concomitantly (N=237), or PNEUMOVAX 23 alone followed 4 weeks later by ZOSTAVAX alone (N=236). At four weeks postvaccination, the varicella-zoster virus (VZV) antibody levels following concomitant use were significantly lower than the VZV antibody levels following nonconcomitant administration (GMTs of 338 vs. 484 gpELISA units/mL, respectively; GMT ratio = 0.70 (95% CI: [0.61, 0.80]).
Limited safety and immunogenicity data from clinical trials are available on the concurrent administration of PNEUMOVAX 23 and vaccines other than ZOSTAVAX.
15. References
- Liang KY, Zeger S. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhyā: The Indian Journal of Statistics (Series B) 2000; 62: 134-148.
16. How is Pneumovax 23 supplied
PNEUMOVAX 23 is supplied as follows:
NDC 0006-4943-00 — a box of 10 single-dose vials, color coded with a purple cap and stripe on the vial labels and cartons.
NDC 0006-4837-03 — a box of 10 single-dose, pre-filled Luer-Lok™ syringes with tip caps, color coded with a violet plunger rod and purple stripe on the syringe labels and cartons.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Inform the patient, parent or guardian of the benefits and risks associated with vaccination.
- Tell the patient, parent or guardian that vaccination with PNEUMOVAX 23 may not offer 100% protection from pneumococcal infection.
- Provide the patient, parent or guardian with the vaccine information statements required by the National Childhood Vaccine Injury Act of 1986, with each immunization.
- Instruct the patient, parent or guardian to report any serious adverse reactions to their health care provider who in turn should report such events to the vaccine manufacturer or the U.S. Department of Health and Human Services through the Vaccine Adverse Event Reporting System (VAERS), 1-800-822-7967, or report online at www.vaers.hhs.gov.
Patient Information about
PNEUMOVAX® 23 (pronounced "noo-mo-vax 23")
Generic Name: pneumococcal vaccine polyvalent
Read this leaflet before you or your child gets the vaccine called PNEUMOVAX 23. If you have any questions about the vaccine after you read this, you should ask your health care provider. This is a summary only. It does not take the place of talking to your doctor, nurse or other health care provider about the vaccine. Only your health care provider can decide if PNEUMOVAX 23 is right for you or your child.
What is PNEUMOVAX 23?
PNEUMOVAX 23 is a vaccine that is given as a shot. It helps protect you from infection by certain germs or bacteria which are called pneumococcus (pronounced "noo-mo-ca-cus"). PNEUMOVAX 23 is for people 50 years of age and older. It is also for people who are 2 years of age and older if they have certain medical conditions that put them at increased risk for infection.
Illnesses or health problems may allow these germs to spread into the blood, lungs, or brain where they can cause serious diseases such as:
- An infection in the blood
- A lung infection (pneumonia) that can also come with an infection in the blood
- An infection of the coverings of the brain and spinal cord (meningitis).
PNEUMOVAX 23 may not protect everyone who gets it. It will not protect against diseases that are caused by bacteria types that are not in the vaccine.
Who should not get PNEUMOVAX 23?
You should not get this vaccine if you (or your child):
- are allergic to any of its ingredients
- had an allergic reaction to PNEUMOVAX 23 in the past
- are less than 2 years old.
What should I tell my health care provider before getting PNEUMOVAX 23?
Tell your health care provider if you (or your child):
- are allergic to PNEUMOVAX 23
- have heart or lung problems
- have a fever
- have immune problems or are receiving radiation treatment for chemotherapy
- are pregnant or breast-feeding
How is PNEUMOVAX 23 given?
Most often, just one shot is given.
If you or your child is in a high-risk group for pneumococcal infection, then your health care provider will decide if it would be helpful to give a second shot of PNEUMOVAX 23 at a later time.
Can PNEUMOVAX 23 be given with other vaccines?
Talk to your health care provider if you plan to get ZOSTAVAX at the same time as PNEUMOVAX 23 because it may be better to get these vaccines at least 4 weeks apart.
Talk to your health care provider if you plan to get PNEUMOVAX 23 at the same time as other vaccines.
What are the possible side effects of PNEUMOVAX 23?
The most common side effects are:
- pain, warmth, soreness, redness, swelling, and hardening at the injection site
- headache
- weakness, feeling tired
- muscle pain
Tell your health care provider or get emergency help right away if you get any of the following problems after vaccination because these may be signs of an allergic reaction or other serious conditions:
- difficulty breathing
- wheezing
- rash
- hives
Side effects at the site where you get the shot may be more common and may feel worse after a second shot than after the first shot.
Tell your health care provider if you or your child has a side effect that bothers you or that does not go away.
For a more complete list of side effects, ask your health care provider.
You may also report any side effect to your or your child's health care provider, or directly to the Vaccine Adverse Event Reporting System (VAERS). You may call the VAERS number 1-800-822-7967 at no charge, or report online to www.vaers.hhs.gov.
What are the ingredients of PNEUMOVAX 23?
| Active Ingredients: | Bacterial sugars from 23 pneumococcal types: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F |
| Inactive Ingredients: | Phenol (a preservative) |
What else should I know about PNEUMOVAX 23?
Some adults and children have problems with leakage of spinal fluid after the skull is cracked or injured or after medical operations and this may increase their risk for pneumococcal infection. PNEUMOVAX 23 may not be able to prevent all of these infections.
The vial stoppers, syringe plunger stopper and syringe tip cap for PNEUMOVAX 23 are not made with natural rubber latex.
This leaflet is a summary of information about PNEUMOVAX 23. If you would like more information, talk to your health care provider or call 1-800-622-4477.
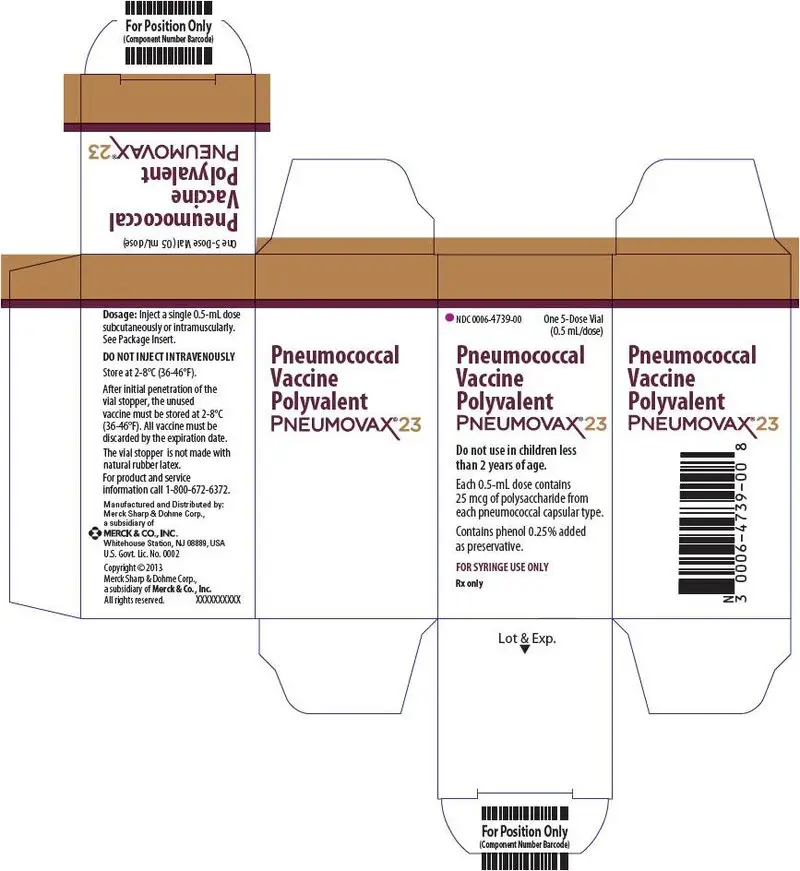
PRINCIPAL DISPLAY PANEL - 5 Dose Vial Carton
NDC 0006-4739-00
One 5-Dose Vial
(0.5 mL/dose)
Pneumococcal
Vaccine
Polyvalent
PNEUMOVAX® 23
Do not use in children less
than 2 years of age.
Each 0.5-mL dose contains
25 mcg of polysaccharide from
each pneumococcal capsular type.
Contains phenol 0.25% added
as preservative.
FOR SYRINGE USE ONLY
Rx only
| PNEUMOVAX 23
pneumococcal vaccine polyvalent injection, solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PNEUMOVAX 23
pneumococcal vaccine polyvalent injection, solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PNEUMOVAX 23
pneumococcal vaccine polyvalent injection, solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |