Drug Class: Laxatives
Highlights of Prescribing Information
PREPOPIK ® (sodium picosulfate, magnesium oxide, and anhydrous citric acid) for oral solution
Initial U.S. Approval: 2012
Recent Major Changes
| Indications and Usage (1) | 08/2018 |
| Dosage and Administration (2.3, 2.4) | 08/2018 |
| Warnings and Precautions (5.1) | 08/2018 |
Indications and Usage for Prepopik
Prepopik is a combination of sodium picosulfate, a stimulant laxative, and magnesium oxide and anhydrous citric acid which form magnesium citrate, an osmotic laxative, indicated for cleansing of the colon as a preparation for colonoscopy in adults and pediatric patients ages 9 years and older. (1)
Prepopik Dosage and Administration
Preparation and Administration
- Each packet of Prepopik must be dissolved with water prior to ingestion and administered according to the dosing regimen. Direct ingestion of the undissolved powder may increase the risk of nausea, vomiting and dehydration. (2.2, 5.8)
- Two doses (one packet per dose) of Prepopik are required for a complete preparation for colonoscopy. The preferred method is the "Split-Dose" method. The alternative is the "Day Before" method. (2.1)
- Additional fluids must be consumed after every dose of Prepopik in both dosing regimens. (2.1, 5.1)
- Do not take oral medications within 1 hour of start of each dose. (2.1, 7.2)
- If taking tetracycline or fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, or penicillamine, take these medications at least 2 hours before and not less than 6 hours after administration of Prepopik. (2.1, 7.3)
- For complete information on preparation before colonoscopy and administration of the dosage regimen, see full prescribing information. (2.1, 2.2, 2.3)
Split-Dose Dosage Regimen (Preferred Method) (2.2)
- First dose: administer during evening before the colonoscopy
- Second dose: administer the next day, during the morning prior to the colonoscopy.
Day-Before Dosage Regimen (Alternative Method), if Split-Dosing is inappropriate (2.3)
- First dose: administer during afternoon or early evening before the colonoscopy.
- Second dose: administer 6 hours later during evening before colonoscopy.
Dosage Forms and Strengths
For oral solution: Each of 2 packets contains 16.1 g of powder for orange flavor or 16.2 g of powder for cranberry flavor : 10 mg sodium picosulfate, 3.5 g magnesium oxide, and 12 g anhydrous citric acid (3)
Contraindications
- Severe renal impairment (creatinine clearance less than 30 mL/minute) (4)
- Gastrointestinal (GI) obstruction or ileus (4)
- Bowel perforation (4)
- Toxic colitis or toxic megacolon (4)
- Gastric retention (4)
- Hypersensitivity to any of the ingredients (4)
Warnings and Precautions
- Risk of fluid and electrolyte abnormalities: Encourage adequate hydration, assess concurrent medications, and consider laboratory assessments prior to and after use. (5.1, 5.2, 7.1)
- Cardiac arrhythmias: Consider pre-dose and post-colonoscopy ECGs in patients at increased risk. (5.2)
- Seizures: Use caution in patients with a history of seizures and patients at increased risk of seizure, including medications that lower the seizure threshold. (5.3, 7.1)
- Patients with mild to moderate renal impairment or taking concomitant medications that affect renal function: Use caution, ensure adequate hydration and consider testing. (4, 5.4, 7.1)
- Mucosal ulcerations: Consider potential for mucosal ulcerations when interpreting colonoscopy findings in patients with known or suspected inflammatory bowel disease. (5.5)
- Suspected GI obstruction or perforation: Rule out diagnosis before administration. (4, 5.6)
- Patients at risk for aspiration: Observe during administration. (5.7)
- Risk of vomiting and other GI complications with ingestion of undissolved powder: Dissolve each packet in 5 ounces of cold water and administered at separate times according to the dosing regimen. (2.3, 2.4, 5.8)
Adverse Reactions/Side Effects
Most common adverse reactions are:
- Adults (>1%): nausea, headache and vomiting. (6.1)
- Pediatrics 9 to 16 years (>5%): nausea, vomiting, and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ferring at 1-888-FERRING (1-888-337-7464) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Drugs that increase risks due to fluid and electrolyte changes. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2018
Full Prescribing Information
1. Indications and Usage for Prepopik
Prepopik® is indicated for cleansing of the colon as a preparation for colonoscopy in adults and pediatric patients 9 years of age and older.
2. Prepopik Dosage and Administration
2.1 Important Preparation and Administration Instructions
- Correct fluid and electrolyte abnormalities before administration of Prepopik [see Warnings and Precautions (5.1)].
- Two doses (one packet per dose) of Prepopik are required for a complete preparation for colonoscopy either as a Split-Dose (preferred) or Day-Before dosing regimen.
- The preferred method is the "Split-Dose" method and consists of two separate doses: the first dose during the evening before the colonoscopy and the second dose the next day, the morning of the day of the colonoscopy [see Dosage and Administration (2.2)].
- The alternative method is the "Day Before" method and consists of two separate doses: the first dose during the afternoon or early evening before the colonoscopy and the second dose 6 hours later during the evening before the colonoscopy [see Dosage and Administration (2.3)].
- Each packet of Prepopik must be dissolved in 5 ounces of cold water prior to ingestion and administered according to the dosing regimen. Direct ingestion of the undissolved powder may increase the risk of nausea, vomiting, dehydration and electrolyte disturbances [see Warnings and Precautions (5.8)].
- Additional fluids must be consumed after every dose of Prepopik in both dosing regimens [see Dosage and Administration (2.2), Warnings and Precautions (5.1)].
- Consume only clear fluids (no solid food) from the start of Prepopik treatment until after the colonoscopy.
- Do not eat solid food or dairy and do not drink anything colored red or purple.
- Do not drink alcohol.
- Do not take other laxatives while taking Prepopik
- Do not take oral medications within one hour before or after starting Prepopik.
- If taking tetracycline or fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, or penicillamine, take these medications at least 2 hours before and not less than 6 hours after administration of Prepopik [see Drug Interactions (7.2)].
- Stop consumption of all fluids at least 2 hours before the colonoscopy.
2.2 Reconstitution of Prepopik Powder
- Reconstitute the Prepopik powder immediately before each administration. Do not prepare the solution in advance.
- Fill the supplied dosing cup with cold water up to the lower (5-ounce) line on the cup and pour in the contents of one packet of Prepopik powder.
- Stir for 2 to 3 minutes. The reconstituted Prepopik solution may become slightly warm as the powder dissolves.
2.3 Split-Dose Dosing Regimen (Preferred Method)
The Split-Dose regimen is the preferred dosing method. The recommended dosage in adults and pediatric patients 9 years of age and older is shown below. Instruct patients to take two separate doses (one packet per dose) in conjunction with fluids.
2.4 Day-Before Dosing Regimen (Alternative Method)
The Day-Before regimen is the alternative dosing method for patients for whom the Split-Dosing is inappropriate. The recommended dosage in adults and pediatric patients 9 years of age and older is shown below. Instruct patients to take two separate doses (one packet per dose) in conjunction with fluids
3. Dosage Forms and Strengths
For oral solution: Each of the two packets contains 10 mg of sodium picosulfate, 3.5 g of magnesium oxide, and 12.0 g of anhydrous citric acid in 16.1g of powder for orange flavor or 16.2 g of powder for cranberry flavor.
4. Contraindications
Prepopik is contraindicated in the following conditions:
- Patients with severe renal impairment (creatinine clearance less than 30 mL/minute) which may result in accumulation of magnesium [see Warnings and Precautions (5.4)]
- Gastrointestinal obstruction or ileus [see Warnings and Precautions (5.6)]
- Bowel perforation [see Warnings and Precautions (5.6)]
- Toxic colitis or toxic megacolon
- Gastric retention
- Hypersensitivity to any of the ingredients in Prepopik [see Adverse Reactions (6.2)]
5. Warnings and Precautions
5.1 Serious Fluid and Electrolyte Abnormalities
Advise patients to hydrate adequately before, during, and after the use of Prepopik. Use caution in patients with congestive heart failure when replacing fluids. If a patient develops significant vomiting or signs of dehydration including signs of orthostatic hypotension after taking Prepopik, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN) and treat accordingly. Approximately 20% of adult patients in both arms (Prepopik, 2L of PEG + E plus two × 5-mg bisacodyl tablets) of clinical trials of Prepopik had orthostatic changes (changes in blood pressure and/or heart rate) on the day of colonoscopy. In adult clinical trials orthostatic changes were documented up to seven days post colonoscopy. In a single study of patients 9 to 16 years of age, approximately 20% of patients in Prepopik arms had orthostatic changes (changes in blood pressure and/or heart rate) compared with approximately 7% of those who received the comparator (PEG) [see Clinical Studies (14)]. These changes occurred up to five days post colonoscopy.
Fluid and electrolyte disturbances can lead to serious adverse reactions including cardiac arrhythmias or seizures and renal impairment. Correct fluid and electrolyte abnormalities before treatment with Prepopik . In addition, use caution when prescribing Prepopik for patients who have conditions or who are using medications that increase the risk for fluid and electrolyte disturbances or that may increase the risk of adverse events of seizure, arrhythmia, and renal impairment . Fluid and electrolyte disturbances can lead to serious adverse reactions including cardiac arrhythmias or seizures and renal impairment. Correct fluid and electrolyte abnormalities before treatment with Prepopik [see Dosage and Administration (2.1)]. In addition, use caution when prescribing Prepopik for patients who have conditions or who are using medications that increase the risk for fluid and electrolyte disturbances or that may increase the risk of adverse events of seizure, arrhythmia, and renal impairment [see Drug Interactions (7.1)].
5.2 Cardiac Arrhythmias
There have been rare reports of serious arrhythmias associated with the use of ionic osmotic laxative products for bowel preparation. Use caution when prescribing Prepopik for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Consider pre-dose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias.
5.3 Seizures
There have been reports of generalized tonic-clonic seizures with the use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities.
Use caution when prescribing Prepopik for patients with a history of seizures and in patients at risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia [see Adverse Reactions (6.2)].
5.4 Use in Patients with Renal Impairment
Prepopik is contraindicated in patients with severe renal impairment (creatinine clearance less than 30 mL/min), accumulation of magnesium in plasma may occur [see Contraindications (4)]. As with other magnesium containing bowel preparations, use caution when prescribing Prepopik for patients with mild to moderate renal impairment or patients taking concomitant medications that may affect renal function (such as diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or non-steroidal anti-inflammatory drugs) [see Drug Interactions (7.1)]. These patients may be at increased risk for renal injury. Advise these patients of the importance of adequate hydration before, during and after the use of Prepopik. Consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients.
5.5 Colonic Mucosal Ulceration, Ischemic Colitis and Ulcerative Colitis
Osmotic laxatives may produce colonic mucosal aphthous ulcerations and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of additional stimulant laxatives with Prepopik may increase this risk. The potential for mucosal ulcerations should be considered when interpreting colonoscopy findings in patients with known or suspected inflammatory bowel disease [see Adverse Reactions (6.2)].
5.6 Use in Patients with Significant Gastrointestinal Disease
If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering Prepopik [see Contraindications (4)]. Use with caution in patients with severe active ulcerative colitis.
5.7 Aspiration
Patients with impaired gag reflex are at risk for regurgitation or aspiration during the administration of Prepopik. Observe these patients during the administration of Prepopik. Use with caution in these patients.
5.8 Risk of Vomiting and Other Gastrointestinal Complications with Ingestion of Undissolved Powder
Each packet must be dissolved in 5 ounces of cold water and administered at separate times according to the dosing regimen [see Dosage and Administration (2.3, 2.4)]. Ingestion of additional water is important to patient tolerance. Direct ingestion of the undissolved powder may increase the risk of nausea, vomiting, dehydration, and electrolyte disturbances.
6. Adverse Reactions/Side Effects
The following serious or otherwise important adverse reactions for bowel preparations are described elsewhere in the labeling:
- Serious Fluid and Electrolyte Abnormalities [see Warnings and Precautions (5.1)]
- Cardiac Arrhythmias [see Warnings and Precautions (5.2)]
- Seizures [see Warnings and Precautions (5.3)]
- Use in Patients with Renal Impairment [see Warnings and Precautions (5.4)]
- Colonic Mucosal Ulceration, Ischemic Colitis and Ulcerative Colitis [see Warnings and Precautions (5.5)]
- Use in Patients with Significant Gastrointestinal Disease [see Warnings and Precautions (5.6)]
- Aspiration [see Warnings and Precautions (5.7)]
- Risk of Vomiting and Other Gastrointestinal Complications with Ingestion of Undissolved Powder [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
In randomized, multicenter, controlled clinical trials, nausea, headache, and vomiting were the most common adverse reactions (>1%) following Prepopik administration. The patients were not blinded to the study drug. Since abdominal bloating, distension, pain/cramping, and watery diarrhea are known to occur in response to colon cleansing preparations, these effects were documented as adverse events in the clinical trials only if they required medical intervention (such as a change in study drug or led to study discontinuation, therapeutic or diagnostic procedures, met the criteria for a serious adverse event), or showed clinically significant worsening during the study that was not in the frame of the usual clinical course, as determined by the investigator.
Prepopik was compared for colon cleansing effectiveness with a preparation containing two liters (2L) of polyethylene glycol plus electrolytes solution (PEG + E) and two 5-mg bisacodyl tablets, all administered the day before the procedure. Table 1 displays the most common adverse reactions in Study 1 and Study 2 for the Prepopik Split-Dose and Day-Before dosing regimens, respectively, each as compared to the comparator preparation.
| Adverse Reaction | Study 1: Split-Dose Regimen | Study 2: Day-Before Regimen | ||
|---|---|---|---|---|
| PREPOPIK (N=305) n (% = n/N) | 2L PEG+E†with 2 × 5-mg bisacodyl tablets (N=298) n (% = n/N) | PREPOPIK (N=296) n (% = n/N) | 2L PEG+E†with 2 × 5-mg bisacodyl tablets (N=302) n (% = n/N) |
|
|
||||
| Nausea | 8 (2.6) | 11 (3.7) | 9 (3.0) | 13 (4.3) |
| Headache | 5 (1.6) | 5 (1.7) | 8 (2.7) | 5 (1.7) |
| Vomiting | 3 (1.0) | 10 (3.4) | 4 (1.4) | 6 (2.0) |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of other oral formulations of sodium picosulfate, magnesium oxide and anhydrous citric acid similar to Prepopik. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity: rash, urticaria, purpura, and anaphylaxis [see Contraindications (4)]
Gastrointestinal: abdominal pain, diarrhea, fecal incontinence, aphthoid ileal ulcers, ischemic colitis [see Warnings and Precautions (5.5)]
Neurologic: generalized tonic-clonic seizures with and without hyponatremia in epileptic patients [see Warnings and Precautions (5.3)].
7. Drug Interactions
7.1 Drugs That May Increase Risks of Fluid and Electrolyte Abnormalities
Use caution when prescribing Prepopik for patients with conditions and/or who are taking other drugs that increase the risk for fluid and electrolyte disturbances or may increase the risk of renal impairment, seizures, arrhythmias, or QT prolongation in the setting of fluid and electrolyte abnormalities [see Warnings and Precautions (5.1, 5.2, 5.3, 5.4)].
7.2 Potential for Reduced Drug Absorption
Prepopik can reduce the absorption of other co-administered drugs [see Dosage and Administration (2.1)]:
- Administer oral medications at least one hour before of the start of administration of Prepopik.
- Administer tetracycline and fluoroquinolone antibiotics [see Drug Interactions (7.3)], iron, digoxin, chlorpromazine, and penicillamine at least 2 hours before and not less than 6 hours after administration of Prepopik.
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of Prepopik have been established for cleansing of the colon as a preparation for colonoscopy in pediatric patients 9 years of age and older. Use of Prepopik in this age group is supported by evidence from adequate and well-controlled trials of Prepopik in adults and a single, dose-ranging, controlled trial in 78 pediatric patients 9 to 16 years of age [see Clinical Studies (14)]. The safety profile of Prepopik in this pediatric population was similar to that seen in adults [see Adverse Reactions (6.1)]. Monitor for possible hypoglycemia in pediatric patients, as Prepopik has no caloric substrate.
The safety and effectiveness of Prepopik in pediatric patients less than 9 years of age have not been established.
8.5 Geriatric Use
Of the 1201 patients in clinical trials who received Prepopik, 215 (18%) patients were 65 years of age or older. No overall differences in safety or effectiveness were observed between geriatric patients and younger patients. However, elderly patients are more likely to have decreased hepatic, renal or cardiac function and may be more susceptible to adverse reactions resulting from fluid and electrolyte abnormalities [see Warnings and Precautions (5.1)].
8.6 Renal Impairment
Prepopik is contraindicated in patients with severe renal impairment (creatinine clearance less than 30 mL/min), as accumulation of magnesium in plasma may occur [see Contraindications (4)]. Patients with mild to moderate renal impairment or patients taking concomitant medications that may affect renal function may be at increased risk for renal injury [see Warnings and Precautions (5.4). Advise these patients of the importance of adequate hydration before, during and after the use of Prepopik [see Dosage and Administration (2.1)]. Consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients.
10. Overdosage
Overdosage of more than the recommended dose of Prepopik may lead to severe electrolyte disturbances, as well as dehydration and hypovolemia, with signs and symptoms of these disturbances [see Warnings and Precautions (5.1)]. Monitor for fluid and electrolyte disturbances and treat symptomatically.
11. Prepopik Description
Prepopik (sodium picosulfate, magnesium oxide and anhydrous citric acid) for oral solution is available in 2 flavors, orange and cranberry flavor, and is provided in two packets. The contents of each is to be dissolved in 5 ounces of cold water and consumed.
Each packet for both flavors contains 10 mg sodium picosulfate, 3.5 g magnesium oxide and 12 g anhydrous citric acid. The product also contains the following inactive ingredients: potassium hydrogen carbonate, saccharine sodium and orange or cranberry flavors. The orange flavor contains acacia gum, lactose, ascorbic acid and butylated hydroxyanisole, and the cranberry flavor contains maltodextrin, glyceryl triacetate (triacetin) and sodium octenyl succinated starch. The following is a description of the three active ingredients:
Sodium picosulfate is a stimulant laxative.
Sodium Picosulfate
- Chemical name: 4,4´-(2-pyridylmethylene) diphenyl bis(hydrogen sulfate) disodium salt, monohydrate
- Chemical formula: C18H13NNa2O8S2.H2O
- Molecular weight: 499.4
- Structural formula:
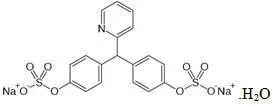
- Sodium picosulfate
Magnesium citrate, which is formed in solution by the combination of magnesium oxide and anhydrous citric acid, is an osmotic laxative.
Magnesium Oxide
- Chemical name: Magnesium oxide
- Chemical formula: Mg O
- Molecular weight: 40.3
- Structural formula: Mg O
Anhydrous Citric Acid
- Chemical name: 2-hydroxypropane-1,2,3-tricarboxylic acid
- Chemical formula: C6H8O7
- Molecular weight: 192.1
- Structural formula:
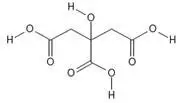
Anhydrous citric acid
12. Prepopik - Clinical Pharmacology
12.1 Mechanism of Action
Sodium picosulfate is hydrolyzed by colonic bacteria to form an active metabolite: bis-(p-hydroxy-phenyl)-pyridyl-2-methane, BHPM, which acts directly on the colonic mucosa to stimulate colonic peristalsis.
Magnesium oxide and citric acid react to create magnesium citrate in solution, which is an osmotic agent that causes water to be retained within the gastrointestinal tract.
12.2 Pharmacodynamics
The stimulant laxative activity of sodium picosulfate together with the osmotic laxative activity of magnesium citrate produces a purgative effect which, when ingested with additional fluids, produces watery diarrhea.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with Prepopik. Sodium picosulfate was not mutagenic in the Ames test, the mouse lymphoma assay and the mouse bone marrow micronucleus test.
In an oral fertility study in rats, Prepopik did not cause any significant adverse effect on male or female fertility parameters up to a maximum dose of 2000 mg/kg twice daily (about 1.2 times the recommended human dose based on the body surface area).
14. Clinical Studies
Adults
The colon cleansing efficacy of Prepopik was evaluated for non-inferiority against a comparator in two randomized, investigator-blinded, active-controlled, multicenter US trials in adult patients scheduled to have an elective colonoscopy. In all, 1195 adult patients were included in the primary efficacy analysis: 601 from Study 1, and 594 from Study 2. Patients ranged in age from 18 to 80 years (mean age 56 years); 61% were female and 39% male. Self-identified race was distributed as follows: 90% White, 10% Black, and less than 1% other. Of these, 3% self-identified their ethnicity as Hispanic or Latino.
Patients randomized to Prepopik in the two studies were treated with one of two dosing regimens:
- In Study 1, Prepopik was given by "Split-Dose" (evening before and day of) dosing, where the first packet was taken the evening before the colonoscopy (between 5:00 and 9:00 PM), followed by five (5) 8-ounce glasses of clear liquid, and the second packet was taken the morning of the colonoscopy (at least 5 hours prior to but no more than 9 hours prior to colonoscopy), followed by three (3) 8-ounce glasses of clear liquid.
- In Study 2, Prepopik was given by "Day-Before" (afternoon/evening before only) dosing, where both packets were taken separately on the day before the colonoscopy, with the first packet taken in the afternoon (between 4:00 and 6:00 PM), followed by five (5) 8-ounce glasses of clear liquid, and the second packet taken in the late evening (approximately 6 hours later, between 10:00 PM and 12:00 AM), followed by three (3) 8-ounce glasses of clear liquid.
The comparator was a preparation containing two liters of polyethylene glycol plus electrolytes solution (PEG + E) and two 5-mg bisacodyl tablets, administered the day before the procedure. All patients in both the Prepopik and comparator groups were limited to a clear liquid diet on the day before the procedure (24 hours before).
The primary efficacy endpoint was the proportion of patients with successful colon cleansing, as assessed by blinded colonoscopists using the Aronchick Scale. The Aronchick scale is a tool used to assess overall colon cleansing. Successful colon cleansing was defined as bowel preparations with >90% of the mucosa seen and mostly liquid stool that were graded excellent (minimal suctioning needed for adequate visualization) or good (significant suctioning needed for adequate visualization) by the colonoscopist.
In both studies, Prepopik was non-inferior to the comparator. In addition, Prepopik provided by Split-Dose dosing met the pre-specified criteria for superiority to the comparator for colon cleansing in Study 1. The comparator in that study was administered entirely on the day prior to colonoscopy. See Tables 3 and 4 below.
| PREPOPIK Split-Dose Regimen | 2L PEG+E* with 2 × 5-mg bisacodyl tablets | Difference between treatment groups | |
|---|---|---|---|
| % (n/N) | % (n/N) | Difference | 95% CI |
|
|||
| 84 % (256/304) | 74% (221/297) | 10% | (3.4%, 16.2%)† |
| PREPOPIK Day-Before Regimen | 2L PEG+E* with 2 × 5-mg bisacodyl tablets | Difference between treatment groups | |
|---|---|---|---|
| % (n/N) | % (n/N) | Difference | 95% CI |
|
|||
| 83% (244/294) | 80% (239/300) | 3 % | (-2.9%, 9.6%)† |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Instruct patients:
- Each packet must be dissolved in 5 ounces of cold water and administered according to the dosing regimen. Direct ingestion of the undissolved powder may increase the risk of nausea, vomiting, dehydration, and electrolyte disturbances [see Warnings and Precautions (5.8).
- Two doses (one packet per dose) of Prepopik are required for a complete preparation for colonoscopy either as a Split-Dose (preferred) or Day-Before dosing regimen.
- Not to take other laxatives while they are taking Prepopik.
- Do not eat solid food or dairy and do not drink anything colored red or purple.
- Do not drink alcohol.
- Do not take oral medications within one hour of starting Prepopik.
- If taking tetracycline or fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, or penicillamine, take these medications at least 2 hours before and not less than 6 hours after administration of Prepopik.
- To follow the directions in the Instructions for Use, for either the Split-Dose or the Day-Before regimen, as prescribed.
- To consume additional fluids after each dose of Prepopik.
- To delay the second dose of Prepopik, if severe bloating, distention, or abdominal pain occurs following the first dose until the symptoms resolve.
- To contact their healthcare provider if they develop significant vomiting or signs of dehydration after taking Prepopik or if they experience altered consciousness (e.g. confusion, delirium, loss of consciousness) or seizures [see Warnings and Precautions (5.1, 5.3, 5.4)].
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Revised: August 2018 | ||
| MEDICATION GUIDE PREPOPIK® (prep-ō-pik) (sodium picosulfate, magnesium oxide, and anhydrous citric acid) for oral solution |
|||
| Read and understand this Medication Guide and the Instructions for Use that come with PREPOPIK at least 2 days before your colonoscopy and again before you start taking Prepopik. | |||
|
What is the most important information I should know about Prepopik? |
|||
| Prepopik and other bowel preparations can cause serious side effects, including: | |||
| Serious loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood. These changes can cause: | |||
|
|||
| Your chance of having fluid loss and changes in blood salts with Prepopik is higher if you: | |||
|
|||
| Tell your healthcare provider right away if you have any of these symptoms of a loss of too much body fluid (dehydration) while taking Prepopik: | |||
|
|
||
| See "What are the possible side effects of Prepopik?" for more information about side effects. | |||
| What is Prepopik? | |||
| Prepopik is a prescription medicine used by adults and children 9 years of age and older, to clean the colon before a colonoscopy. Prepopik cleans your colon by causing you to have diarrhea. Cleaning your colon helps your healthcare provider see the inside of your colon more clearly during your colonoscopy. | |||
| It is not known if Prepopik is safe and effective in children under 9 years of age. | |||
| Do not take Prepopik if your healthcare provider has told you that you have: | |||
|
|||
| Before taking Prepopik, tell your healthcare provider about all of your medical conditions, including if you: | |||
|
|||
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |||
| Prepopik may affect how other medicines work, and other medicines may affect how Prepopik works. Medicines taken by mouth may not be absorbed properly when taken within 1 hour before the start of Prepopik. | |||
| Especially tell your healthcare provider if you take: | |||
|
|||
| The following medicines should be taken at least 2 hours before starting Prepopik and not less than 6 hours after taking Prepopik: | |||
|
|
||
| Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure if you are taking the medicines listed above. | |||
| Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | |||
| How should I take Prepopik? | |||
| See the Instructions for Use for dosing instructions. You must read, understand, and follow these instructions to take Prepopik the right way. | |||
|
|||
| Contact your healthcare provider right away if after taking Prepopik you have severe vomiting, signs of dehydration, changes in consciousness such as feeling confused, delirious or fainting (loss of consciousness) or seizures after taking Prepopik. | |||
| Prepopik can cause serious side effects, including: | |||
|
|||
|
|
|
|
|
|||
| The most common side effects of Prepopik in adults include: | |||
|
|
| |
| The most common side effects of Prepopik in children 9 to 16 years of age include: | |||
|
|
| |
| These are not all the possible side effects of Prepopik. | |||
| Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
| How should I store Prepopik? | |||
|
|||
| Keep Prepopik and all medicines out of the reach of children. | |||
| General information about the safe and effective use of Prepopik. | |||
| Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Prepopik for a condition for which it was not prescribed. Do not give Prepopik to other people, even if they are going to have the same procedure you are. It may harm them. You can ask your pharmacist or healthcare provider for information about Prepopik that is written for health professionals. | |||
| What are the ingredients in Prepopik? | |||
| Prepopik comes in a carton containing 2 packets, each containing 16.1 grams of powder in orange flavor or 16.2 grams of powder in cranberry flavor, along with a pre-marked dosing cup. | |||
| Active ingredients: sodium picosulfate, magnesium oxide, and anhydrous citric acid | |||
| Inactive ingredients: potassium hydrogen carbonate, saccharin sodium, orange flavor which contains acacia gum, lactose, ascorbic acid and butylated hydroxyanisole or the cranberry flavor which contains maltodextrin, glyceryl triacetate (triacetin) and sodium octenyl succinated starch. | |||
| Manufactured for: Ferring Pharmaceuticals Inc. Parsippany, NJ 07054, USA For more information, go to www.ferring.com or call 1-888-337-7464. |
|||
INSTRUCTIONS FOR USE
Prepopik® (prep- o-pik)
(sodium picosulfate, magnesium oxide, and anhydrous citric acid)
for oral solution
Before Taking Prepopik
There are 2 different methods for taking Prepopik. It is better (preferred) to use the Split-Dose method. If you cannot use the Split-Dose method, you can take Prepopik using the Day-Before method. Talk with your healthcare provider before you start if you have any questions.
- Start a clear-liquid diet the day before your colonoscopy. Only drink clear liquids all day the day before your colonoscopy, and the next day until 2 hours before your colonoscopy. Stop drinking all fluids at least 2 hours before the colonoscopy.
- You must drink enough clear liquids to keep your body hydrated for the entire day before your colonoscopy.
Important:
See Table 1 for a list of liquids you can drink for your clear liquid diet.
|
Table 1: List of liquids for the clear-liquid diet
|
Important:
See Table 2 for the items you cannot eat or drink before your colonoscopy.
|
Table 2: Do not eat or drink these items during the clear-liquid diet
|
Important:
- Prepopik is a powder that must be added to cold water right before use.
- Do not prepare the solution ahead of time. Take Prepopik right after it is prepared.
- Do not swallow the powder without adding it to cold water first.
Split-Dose Instructions
Dose 1 – In the evening the day before your colonoscopy (sometime between 5:00 PM to 9:00 PM)
|  |
- Stir for 2 to 3 minutes to dissolve the powder. Use a clock or timer The dosing cup may feel slightly warm as the powder dissolves. This is normal.
- Drink all the solution right away.
Note: Prepopik powder should be mixed in cold water immediately before use. Do not prepare the solution ahead of time or store the solution for later use. Do not refrigerate or add ice to the solution.
- Follow this Prepopik dose by drinking at least five 8 ounce cups of clear liquid (using the upper line on the dosing cup provided- see figure below) over the next 5 hours.
- After taking Prepopik if you have any bloating or feeling like your stomach is upset, wait to take your second dose until your stomach feels better.
Important: See Table 1 for a list of acceptable clear liquids.
Dose 2 - In the morning of colonoscopy (approximately 5 hours prior to colonoscopy)
Note: Do not eat solid food. Drink only clear liquids.
|  |
- Open the second packet of Prepopik and add all the powder to the cold water in the dosing cup.
- Stir for 2 to 3 minutes to dissolve the powder. Use a clock or timer.
- Drink all the solution right away.
Note: Prepopik powder should be mixed in cold water immediately before use. Do not prepare the solution ahead of time or store the solution for later use. Do not refrigerate or add ice to the solution.
- Follow this Prepopik dose by drinking at least three 8 ounce cups of clear liquids (using the upper line on the dosing cup- see figure below). You can continue to drink clear liquids up to 2 hours before the colonoscopy.
Important: See Table 1 for a list of acceptable clear liquids.
Stop drinking clear liquids 2 hours before your colonoscopy, or as advised by your healthcare provider.
| Day-Before Instructions | |
| Dose 1 – In the afternoon or early evening the day before your colonoscopy (sometime between 4:00 PM to 6:00 PM) | |
|  |
- Open 1 packet of Prepopik and add all the powder to the cold water in the dosing cup. To open the Prepopik packet cut along the dotted line at the top of the packet using scissors.
- Stir for 2 to 3 minutes to dissolve the powder. Use a clock or timer. The dosing cup may feel slightly warm as the powder dissolves. This is normal.
- Drink all the solution right away.
Note: Prepopik powder should be mixed in cold water immediately before use. Do not prepare the solution ahead of time or store the solution for later use. Do not refrigerate or add ice to the solution.
- Follow this Prepopik dose by drinking at least five 8 ounce cups of clear liquids (using the upper line on the dosing cup- see figure below) over the next 5 hours.
After taking Prepopik if you have any bloating or feeling like your stomach is upset, wait to take your second dose until your stomach feels better.
Important: See Table 1 for a list of acceptable clear liquids.
Dose 2 – In the evening before your colonoscopy (sometime between 10:00 PM to 12:00 AM):
| Note: Do not eat solid food. Drink only clear liquids. | |
|  |
- Open the second packet of Prepopik and add all of the powder to the cold water in the dosing cup.Stir for 2 to 3 minutes to dissolve the powder. Use a clock or timer.
- Drink all of this solution right away.
Note: Prepopik powder should be mixed in cold water immediately before use. Do not prepare the solution ahead of time or store the solution for later use. Do not refrigerate or add ice to the solution.
- Follow this Prepopik dose by drinking at least three 8 ounce cups of clear liquids (using the upper line on the dosing cup- see figure below) over the next 5 hours.
Important: See Table 1 for a list of acceptable clear liquids.
Stop drinking clear liquids 2 hours before your colonoscopy, or as advised by your healthcare provider.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: August 2018
| PREPOPIK
sodium picosulfate, magnesium oxide, and anhydrous citric acid powder, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PREPOPIK
sodium picosulfate, magnesium oxide, and anhydrous citric acid powder, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ferring Pharmaceuticals Inc. (103722955) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ferring Production Inc. | 079510999 | LABEL(55566-9300, 55566-9700) , PACK(55566-9300, 55566-9700) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ferring Pharmaceuticals (China) Co., Ltd. | 527903976 | MANUFACTURE(55566-9300, 55566-9700) , PACK(55566-9300, 55566-9700) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jungbunzlauer Ladenburg GmbH | 322121609 | API MANUFACTURE(55566-9300, 55566-9700) , ANALYSIS(55566-9300, 55566-9700) , PACK(55566-9300, 55566-9700) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cambrex Profarmaco Milano S.R.L. | 438051401 | API MANUFACTURE(55566-9300, 55566-9700) , PACK(55566-9300, 55566-9700) , ANALYSIS(55566-9300, 55566-9700) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Tomita Phamaceutical Co., Ltd. | 690643499 | API MANUFACTURE(55566-9300, 55566-9700) , PACK(55566-9300, 55566-9700) , ANALYSIS(55566-9300, 55566-9700) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| F.M. Howell & Company | 962888116 | PACK(55566-9300, 55566-9700) | |