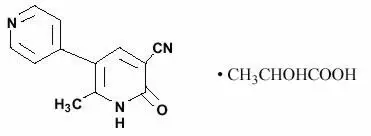
Drug Detail:Milrinone (Milrinone [ mil-ri-none ])
Drug Class: Inotropic agents
Primacor Dosage and Administration
PRIMACOR (milrinone lactate) should be administered with a loading dose followed by a continuous infusion (maintenance dose) according to the following guidelines:
MAINTENANCE DOSE
| Infusion Rate | Total Daily Dose
(24 Hours) | ||
|---|---|---|---|
| Minimum | 0.375 mcg/kg/min | 0.59 mg/kg | Administer as a |
| Standard | 0.50 mcg/kg/min | 0.77 mg/kg | continuous |
| Maximum | 0.75 mcg/kg/min | 1.13 mg/kg | intravenous infusion |
The infusion rate should be adjusted according to hemodynamic and clinical response. Patients should be closely monitored. In controlled clinical studies, most patients showed an improvement in hemodynamic status as evidenced by increases in cardiac output and reductions in pulmonary capillary wedge pressure.
Note: See"Dosage Adjustment in Renally Impaired Patients."Dosage may be titrated to the maximum hemodynamic effect and should not exceed 1.13 mg/kg/day. Duration of therapy should depend upon patient responsiveness.
The maintenance dose in mL/hr by patient body weight (kg) may be determined by reference to the following table.
Note:PRIMACOR supplied in 100 mL and 200 mL Flexible Containers (200 mcg/mL in 5% Dextrose Injection) need not be diluted prior to use.
| Maintenance Dose | Patient Body Weight (kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mcg/kg/min) | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 |
| 0.375 | 3.4 | 4.5 | 5.6 | 6.8 | 7.9 | 9.0 | 10.1 | 11.3 | 12.4 | 13.5 |
| 0.400 | 3.6 | 4.8 | 6.0 | 7.2 | 8.4 | 9.6 | 10.8 | 12.0 | 13.2 | 14.4 |
| 0.500 | 4.5 | 6.0 | 7.5 | 9.0 | 10.5 | 12.0 | 13.5 | 15.0 | 16.5 | 18.0 |
| 0.600 | 5.4 | 7.2 | 9.0 | 10.8 | 12.6 | 14.4 | 16.2 | 18.0 | 19.8 | 21.6 |
| 0.700 | 6.3 | 8.4 | 10.5 | 12.6 | 14.7 | 16.8 | 18.9 | 21.0 | 23.1 | 25.2 |
| 0.750 | 6.8 | 9.0 | 11.3 | 13.5 | 15.8 | 18.0 | 20.3 | 22.5 | 24.8 | 27.0 |
When administering PRIMACOR (milrinone lactate) by continuous infusion, it is advisable to use a calibrated electronic infusion device.
The Flexible Container has a concentration of milrinone equivalent to 200 mcg/mL in 5% Dextrose Injection. To use the Flexible Container, tear the overwrap at the notch and remove the Pre-Mix solution container. Squeeze the container firmly to check for leaks. Discard the container if leaks are found since the sterility of the product could be affected. Do not add supplementary medication. To prepare the container for administration of PRIMACOR intravenously, use aseptic techniques.
- 1)
- The flow control clamp of the administration set is closed.
- 2)
- The cover of the outlet port at the bottom of the container is removed.
- 3)
- Noting the full directions on the administration set carton, the piercing pin of the set is inserted into the port with a twisting motion until it is firmly seated.
- 4)
- The container is suspended on the hanger.
- 5)
- The drip chamber is squeezed and released to establish the fill level.
- 6)
- The flow control clamp is opened to expel air from the set, and then closed.
- 7)
- The set is attached to the venipuncture device, primed, and if not indwelling, the venipuncture is performed.
- 8)
- The rate of administration is controlled with the flow control clamp. WARNING- DO NOT USE IN SERIES CONNECTIONS. Caution: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete.
Intravenous drug products should be inspected visually and should not be used if particulate matter or discoloration is present.
Dosage Adjustment in Renally Impaired Patients
Data obtained from patients with severe renal impairment (creatinine clearance = 0 to 30 mL/min) but without congestive heart failure have demonstrated that the presence of renal impairment significantly increases the terminal elimination half-life of PRIMACOR. Reductions in infusion rate may be necessary in patients with renal impairment. For patients with clinical evidence of renal impairment, the recommended infusion rate can be obtained from the following table:
| Creatinine Clearance (mL/min/1.73m2) | Infusion Rate (mcg/kg/min) |
|---|---|
| 5 | 0.20 |
| 10 | 0.23 |
| 20 | 0.28 |
| 30 | 0.33 |
| 40 | 0.38 |
| 50 | 0.43 |
| PRIMACOR
milrinone lactate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC |