Drug Detail:Pulmozyme (Dornase alfa (inhalation) [ door-nase-al-fa ])
Drug Class: Miscellaneous respiratory agents
Highlights of Prescribing Information
PULMOZYME® (dornase alfa) inhalation solution, for inhalation use
Initial U.S. Approval: 1993
Recent Major Changes
| Dosage and Administration (2.2) | 07/2021 |
Indications and Usage for Pulmozyme
PULMOZYME is a recombinant DNase enzyme indicated in conjunction with standard therapies for the management of cystic fibrosis (CF) patients to improve pulmonary function. (1)
Pulmozyme Dosage and Administration
- The recommended dosage is one 2.5 mg single-use ampule inhaled once daily using a recommended nebulizer. (2.1)
- Some patients may benefit from twice daily administration. (2.1)
Dosage Forms and Strengths
Inhalation solution: 2.5 mg/2.5 mL clear, colorless solution in single-dose ampules. (3)
Contraindications
PULMOZYME is contraindicated in patients with known hypersensitivity to dornase alfa, Chinese Hamster Ovary cell products, or any component of the product. (4)
Warnings and Precautions
None. (5)
Adverse Reactions/Side Effects
The most common adverse reactions (occurring in ≥3% of patients treated with PULMOZYME over placebo) seen in clinical trials in CF patients were: voice alteration, pharyngitis, rash, laryngitis, chest pain, conjunctivitis, rhinitis, decrease in FVC of ≥10%, fever, and dyspnea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2021
Full Prescribing Information
1. Indications and Usage for Pulmozyme
PULMOZYME® is indicated, in conjunction with standard therapies, for the management of pediatric and adult patients with cystic fibrosis (CF) to improve pulmonary function.
In CF patients with an FVC ≥ 40% of predicted, daily administration of PULMOZYME has also been shown to reduce the risk of respiratory tract infections requiring parenteral antibiotics.
2. Pulmozyme Dosage and Administration
2.1 Recommended Dosage
The recommended dosage, in most cystic fibrosis patients, is one 2.5 mg single-dose ampule inhaled once daily using a recommended jet nebulizer connected to an air compressor system or eRapid™ Nebulizer System.
Some patients may benefit from twice daily administration [see Clinical Studies (14)].
2.2 Administration Instructions
Nebulizer Information
Administer PULMOZYME via the eRapid Nebulizer System or via a jet nebulizer connected to an air compressor with an adequate air flow and equipped with a mouthpiece or suitable face mask (see Table 1). No data are currently available to support the administration of PULMOZYME with other nebulizer systems.
When PULMOZYME is administered with the eRapid Nebulizer System, replace the handset after 90 uses, regardless of whether the EasyCare cleaning aid is used. Since delivery data are not available for PULMOZYME administered with the eRapid handset beyond 90 administrations, delivery of the appropriate therapeutic dose of PULMOZYME cannot be assured beyond 90 administrations. The eRapid Nebulizer System should only be used by adults and pediatric patients who can use a mouthpiece, and not by younger patients who need a mask to take PULMOZYME.
The patient should follow the manufacturer's instructions on the use and maintenance of the equipment, including cleaning and disinfection procedures.
For additional information, refer to recommended nebulizer manufacturers' Instructions for Use.
| Jet Nebulizer* | Compressor |
|---|---|
|
|
| Hudson T Up-draft II® | Pulmo-Aide® or legally marketed compressor of identical pressure and flow rate (maximum 30 psi, 12 LPM). |
| Marquest Acorn II® | |
| PARI LC® Plus | PARI PRONEB® or legally marketed compressor of identical pressure and flow rate (maximum 24 psi, 9 LPM). |
| †PARI BABY™ | |
| Durable Sidestream® | MOBILAIRE™, Porta-NEB® or legally marketed compressor of identical pressure and flow rate (maximum 45 psi, 7 LPM). |
| Nebulizer System | |
| eRapid™ Nebulizer System‡ | |
3. Dosage Forms and Strengths
Inhalation solution: 2.5 mg/2.5 mL clear, colorless solution in single-dose ampules.
4. Contraindications
PULMOZYME is contraindicated in patients with known hypersensitivity to dornase alfa, Chinese Hamster Ovary cell products, or any component of the product.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to PULMOZYME in 902 patients, with exposures ranging from 2 weeks of daily administration up to once or twice daily administration for six months. PULMOZYME was studied in both placebo-controlled and uncontrolled trials (n=804 and n=98). The population of patients in placebo-controlled trials was with FVC ≥ 40% of predicted (n=643) or with more advanced pulmonary disease, FVC < 40% of predicted (n=161). The population in the uncontrolled trial included 98 pediatric patients with CF ranging from 3 months to 10 years of age. More than half of the patients received PULMOZYME 2.5 mg by inhalation once a day (n=581), while the rest of patients (n=321) received PULMOZYME 2.5 mg by inhalation twice a day.
Placebo-Controlled Trials
Trial 2: Trial 2 was a randomized, placebo-controlled trial in patients with more advanced pulmonary disease (FVC < 40% of predicted) who were treated for 12 weeks. In this trial, the safety profile of PULMOZYME was similar to that reported in patients with less advanced pulmonary disease (FVC ≥ 40% of predicted). Adverse reactions that were reported in this trial with a higher proportion (greater than 3%) in the PULMOZYME treated patients are listed in Table 2.
| Adverse Reactions (of any severity or seriousness) | Trial 1 CF Patients with FVC ≥ 40% of predicted treated for 24 weeks | Trial 2 CF Patients with FVC <40% of predicted treated for 12 weeks |
|||
|---|---|---|---|---|---|
| Placebo n=325 | Pulmozyme QD n=322 | Pulmozyme BID n=321 | Placebo n=159 | Pulmozyme QD n=161 |
|
|
|||||
| Voice alteration | 7% | 12% | 16% | 6% | 18% |
| Pharyngitis | 33% | 36% | 40% | 28% | 32% |
| Rash | 7% | 10% | 12% | 1% | 3% |
| Laryngitis | 1% | 3% | 4% | 1% | 3% |
| Chest Pain | 16% | 18% | 21% | 23% | 25% |
| Conjunctivitis | 2% | 4% | 5% | 0% | 1% |
| Rhinitis | Differences were less than 3% | 24% | 30% | ||
| FVC decrease of ≥ 10% of predicted* | 17% | 22% | |||
| Fever | 28% | 32% | |||
| Dyspepsia | 0% | 3% | |||
| Dyspnea (when reported as serious) | Differences were less than 3% | 12%† | 17%† | ||
Mortality rates observed in controlled trials were similar for the placebo and PULMOZYME treated patients. Causes of death were consistent with progression of cystic fibrosis and included apnea, cardiac arrest, cardiopulmonary arrest, cor pulmonale, heart failure, massive hemoptysis, pneumonia, pneumothorax, and respiratory failure.
7. Drug Interactions
Available data indicate there are no clinically important drug-drug interactions with PULMOZYME.
8. Use In Specific Populations
8.1 Pregnancy
Risk summary
There are no adequate and well-controlled studies with PULMOZYME in pregnant women. However, animal reproduction studies have been conducted with dornase alfa. In these studies, no evidence of fetal harm was observed in rats and rabbits at doses of dornase alfa up to approximately 600 times the maximum recommended human dose (MRHD).
The background risk of major birth defects and miscarriage for the cystic fibrosis population is unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
8.4 Pediatric Use
The safety and effectiveness of PULMOZYME in conjunction with standard therapies for cystic fibrosis have been established in pediatric patients. Use of PULMOZYME in pediatric patients is supported by evidence in the following age groups:
- Patients 5 to 17 years of age: Use of PULMOZYME in patients 5 to 17 years of age is supported by evidence from a randomized, placebo-controlled trial of 303 of clinically stable cystic fibrosis patients 5 to 17 years of age who received PULMOZYME [see Clinical Studies (14)].
- Patients less than 5 years: Use of PULMOZYME in patients less than 5 years of age is supported by extrapolation of efficacy data in patients 5 years of age and older with additional safety data in 65 pediatric patients aged 3 months to less than 5 years who received PULMOZYME 2.5 mg daily by inhalation for 2 weeks [see Adverse Reactions (6) and Clinical Studies (14)].
11. Pulmozyme Description
Dornase alfa is a recombinant human deoxyribonuclease I (rhDNase), an enzyme which selectively cleaves DNA. The protein is produced by genetically engineered Chinese Hamster Ovary (CHO) cells containing DNA encoding for the native human protein, deoxyribonuclease I (DNase). The product is purified by column chromatography and tangential flow filtration. The purified glycoprotein contains 260 amino acids with an approximate molecular weight of 37,000 daltons. The primary amino acid sequence is identical to that of the native human enzyme.
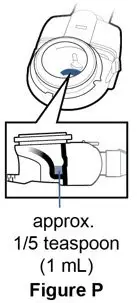
PULMOZYME (dornase alfa) inhalation solution is administered by inhalation of an aerosol mist produced by a compressed air driven nebulizer or an approved nebulizer system [see Clinical Studies (14) and Dosage and Administration (2)]. PULMOZYME is a sterile, clear, colorless, highly purified solution in single-dose ampules. Each ampule delivers 2.5 mL of the solution to the nebulizer bowl. Each mL of aqueous solution contains 1 mg dornase alfa, calcium chloride dihydrate (0.15 mg) and sodium chloride (8.77 mg). The solution contains no preservative. The nominal pH of the solution is 6.3.
12. Pulmozyme - Clinical Pharmacology
12.1 Mechanism of Action
PULMOZYME is recombinant human deoxyribonuclease I (rhDNase), an enzyme which selectively cleaves DNA. In preclinical in vitro studies, PULMOZYME hydrolyzes the DNA in sputum of CF patients and reduces sputum viscoelasticity. In CF patients, retention of viscous purulent secretions in the airways contributes both to reduced pulmonary function and to exacerbations of infection. Purulent pulmonary secretions contain very high concentrations of extracellular DNA released by degenerating leukocytes that accumulate in response to infection.
12.3 Pharmacokinetics
When 2.5 mg PULMOZYME was administered by inhalation to eighteen CF patients, mean sputum concentrations of 3 µg/mL DNase were measurable within 15 minutes. Mean sputum concentrations declined to an average of 0.6 µg/mL two hours following inhalation. Inhalation of up to 10 mg TID of PULMOZYME by 4 CF patients for six consecutive days did not result in a significant elevation of serum concentrations of DNase above normal endogenous levels. After administration of up to 2.5 mg of PULMOZYME twice daily for six months to 321 CF patients, no accumulation of serum DNase was noted. Dornase alfa is expected to be metabolized by proteases present in biological fluids. A human intravenous dose study suggested an elimination half-life of 3-4 hours for dornase alfa.
PULMOZYME, 2.5 mg by inhalation, was administered daily to 98 patients aged 3 months to ≤ 10 years, and bronchoalveolar lavage (BAL) fluid was obtained within 90 minutes of the first dose. BAL DNase concentrations were detectable in all patients but showed a broad range, from 0.007 to 1.8 µg/mL. Over an average of 14 days of exposure, serum DNase concentrations (mean ± s.d.) increased by 1.1 ± 1.6 ng/mL for the 3 months to < 5 year age group and by 0.8 ± 1.2 ng/mL for the 5 to ≤ 10 year age group. The relationship between BAL or serum DNase concentration and adverse experiences and clinical outcomes is unknown.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
PULMOZYME produced no treatment-related increases in the incidence of tumors in a lifetime study in Sprague Dawley rats that were administered inhaled doses up to 0.246 mg/kg/day (approximately 30 times the MRHD in adults). There was no increase in the development of benign or malignant neoplasms and no occurrence of unusual tumor types in rats after lifetime exposure.
PULMOZYME tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitro mouse lymphoma assay, and in vivo mouse bone marrow micronucleus assay. No evidence of impairment of fertility was observed in male and female rats that received intravenous doses up to 10 mg/kg/day (approximately 600 times the MRHD in adults).
14. Clinical Studies
Trial in CF Patients with FVC ≥40% of Predicted
PULMOZYME has been evaluated in a randomized, placebo-controlled trial of clinically stable cystic fibrosis patients, 5 years of age and older, with baseline forced vital capacity (FVC) greater than or equal to 40% of predicted and receiving standard therapies for cystic fibrosis. Patients were treated with placebo (325 patients), 2.5 mg of PULMOZYME once a day (322 patients), or 2.5 mg of PULMOZYME twice a day (321 patients) for six months administered via a Hudson T Up-draft II® nebulizer with a Pulmo-Aide® compressor.
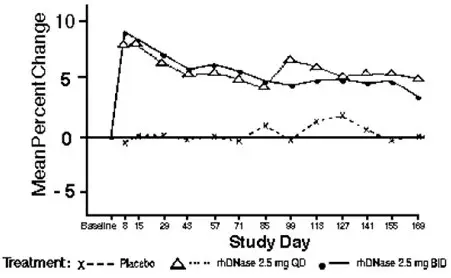
Both doses of PULMOZYME resulted in significant reductions in the number of patients experiencing respiratory tract infections requiring use of parenteral antibiotics compared with the placebo group. Administration of PULMOZYME reduced the relative risk of developing a respiratory tract infection by 27% and 29% for the 2.5 mg daily dose and the 2.5 mg twice daily dose, respectively (see Table 3). The data suggest that the effects of PULMOZYME on respiratory tract infections in older patients ( > 21 years) may be smaller than in younger patients, and that twice daily dosing may be required in the older patients. Patients with baseline FVC > 85% may also benefit from twice a day dosing (see Table 3). The reduced risk of respiratory infection observed in PULMOZYME treated patients did not directly correlate with improvement in FEV1 during the initial two weeks of therapy.
Within 8 days of the start of treatment with PULMOZYME, mean FEV1 increased 7.9% in those treated once a day and 9.0% in those treated twice a day compared to the baseline values. The overall mean FEV1 during long-term therapy increased 5.8% from baseline at the 2.5 mg daily dose level and 5.6% from baseline at the 2.5 mg twice daily dose level. Placebo recipients did not show significant mean changes in pulmonary function testing (see Figure 1).
For patients 5 years of age or older with baseline FVC greater than or equal to 40%, administration of PULMOZYME decreased the incidence of occurrence of first respiratory tract infection requiring parenteral antibiotics, and improved mean FEV1, regardless of age or baseline FVC.
| Placebo N=325 | 2.5 mg QD N=322 | 2.5 mg BID N=321 |
|
| Percent of Patients Infected | 43% | 34% | 33% |
| Relative Risk (vs placebo) | 0.73 | 0.71 | |
| p-value (vs placebo) | 0.015 | 0.007 | |
| Subgroup by Age and Baseline FVC | Placebo % (N) | 2.5 mg QD % (N) | 2.5 mg BID % (N) |
| Age | |||
| 5-20 years | 42% (201) | 25% (199) | 28% (184) |
| 21 years and older | 44% (124) | 48% (123) | 39% (137) |
| Baseline FVC | |||
| 40-85% Predicted | 54% (194) | 41% (201) | 44% (203) |
| > 85% Predicted | 27% (131) | 21% (121) | 14% (118) |
 |
16. How is Pulmozyme supplied
PULMOZYME (dornase alfa) inhalation solution is a sterile, clear, colorless solution supplied in:
- 30 unit cartons containing 5 foil pouches of 6 single-dose ampules. Each 2.5 mL ampule contains 2.5 mg of dornase alfa (1 mg/mL): NDC 50242-100-40.
17. Patient Counseling Information
Advise patients to read the FDA-approved Patient Labeling (Instructions for Use).
INSTRUCTIONS FOR USE
PULMOZYME® (PULL-muh-zyme)
(dornase alfa)
Inhalation Solution
This Instructions for Use contains information on how to use PULMOZYME with Jet Nebulizers and Compressors
See the other side of this Instructions for Use for information on use of Pulmozyme with the ultrasonic eRapid Nebulizer System
Read this Instructions for Use before you start taking Pulmozyme and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
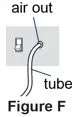
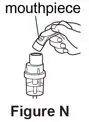
A nebulizer and a compressor are used together to give a dose of Pulmozyme. A nebulizer changes the Pulmozyme liquid medicine into a fine mist you inhale by breathing through a mouthpiece. A compressor gives the nebulizer power and makes the nebulizer work.
Pulmozyme should only be used with the approved nebulizers and appropriate compressors as recommended below, or with the eRapid Nebulizer System (see other side of this Instructions for Use). Read and follow the manufacturer's instructions.
Do not use any other inhaled medicines in the nebulizer at the same time. Keep all other inhaled medicine systems completely separate from Pulmozyme.
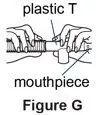
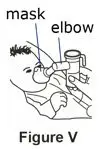
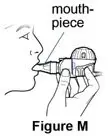
Use the mouthpiece or face mask provided with the nebulizer kit.
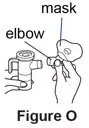
If your child cannot breathe in or breathe out by mouth, you may use the PARI BABY reusable nebulizer, but you should discuss it with your doctor first. The PARI BABY nebulizer is the same as the PARI LC Plus Jet system, except the mouthpiece is replaced by a tight-fitting face mask connected to an elbow piece.
Follow the steps on this side of the sheet to give Pulmozyme using the following jet nebulizer systems
| Nebulizer | Compressor |
|---|---|
| Hudson T Up-draft II | A compressor with the following specifications is recommended:
|
| Marquest Acorn II | |
| PARI LC Plus | |
| PARI BABY | |
| Durable Sidestream |
For additional information on an appropriate compressor to use with Pulmozyme, read the manufacturer's instructions for the recommended nebulizer.
Important Information You Need to Know Before Using PULMOZYME
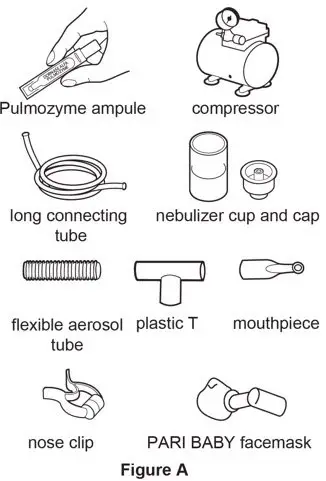
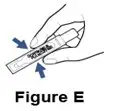
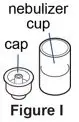
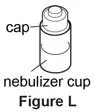
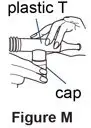
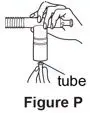
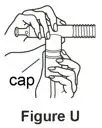
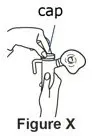
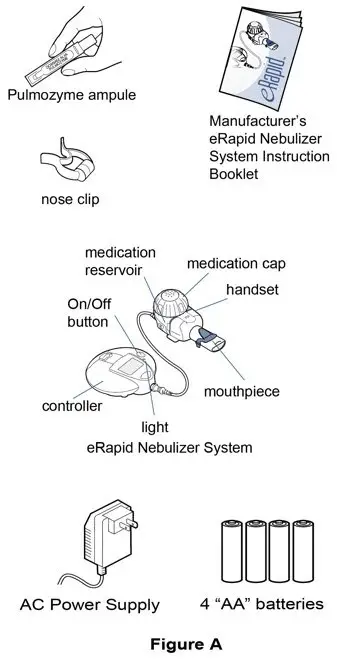
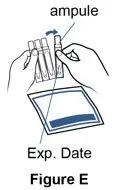
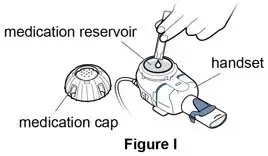
Supplies you will need to give a dose of Pulmozyme (See Figure A):
- 1 Pulmozyme ampule
- Compressor
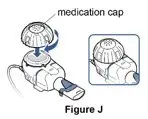
- Nebulizer cup and cap (screw-on or snap-on)
- Plastic T (not needed for Sidestream nebulizer or PARI BABY)
- Flexible aerosol tube (not needed for Sidestream nebulizer or PARI BABY)
- Mouthpiece (clean) or PARI BABY facemask
- Long connecting tube
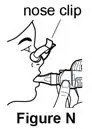
- Nose clip (optional, not needed for PARI BABY)
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
US License No. 1048
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 04/2022
INSTRUCTIONS FOR USE
PULMOZYME® (PULL-muh-zyme)
(dornase alfa)
Inhalation Solution
This Instructions for Use contains information on how to use PULMOZYME with the eRapid Nebulizer System
See the other side of this Instructions for Use for information on use with Jet Nebulizers and Compressors
Read this Instructions for Use before you start taking Pulmozyme and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
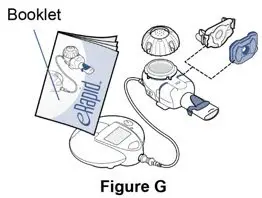
This information does not take the place of the Manufacturer's eRapid Nebulizer System Instruction Booklet. This information is needed to show you the right way to use the eRapid Nebulizer System.
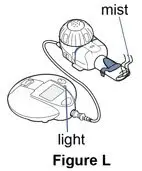
The eRapid Nebulizer System changes the Pulmozyme liquid medicine into a fine mist you inhale by breathing through a mouthpiece.
Do not use any other inhaled medicines in the nebulizer at the same time. Keep all other inhaled medication systems completely separate from Pulmozyme.
The eRapid Nebulizer System should only be used by adults and children who can use a mouthpiece, and not by younger children who need a mask to take Pulmozyme.
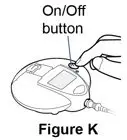
Follow the instructions on this side of the sheet to give Pulmozyme using the eRapid Nebulizer System.
Important Information You Need to Know Before Using PULMOZYME
Supplies you will need to give a dose of Pulmozyme (See Figure A):
- 1 Pulmozyme ampule
- eRapid Nebulizer System, including the:
- eRapid Nebulizer Handset (handset)
- eBase Controller (controller)
- Power source for the controller, using either:
- 4 "AA" batteries (disposable or rechargeable)
- or an AC Power Supply plugged into a typical wall outlet (110-volt power outlet)
- Nose clip (optional)
- Manufacturer's eRapid Nebulizer System Instruction Booklet
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
US License No. 1048
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 04/2022
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
| PULMOZYME
dornase alfa solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Genentech, Inc. (080129000) |
| Registrant - Genentech, Inc. (080129000) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Singapore Technical Operations Pte. Ltd. (RSTO) | 937189173 | ANALYSIS(50242-100) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Diagnostics GmbH | 323105205 | ANALYSIS(50242-100) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc. | 080129000 | ANALYSIS(50242-100) , MANUFACTURE(50242-100) , API MANUFACTURE(50242-100) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc | 146373191 | ANALYSIS(50242-100) | |